Radial Electron Probability Density Function of Atoms.
Introduction
In quantum mechanics free particles are described by wave functions which satisfy a wave equation. So, there is a wave-particle duality with a relationship between particle properties (energy, E, and momentum, p) and wave properties (frequency, ν, and wavelength, λ) given by Plank's law (E = h ν) and de Broglie equation (λ = h p ), both of which involve the Planck's constant, h.
The wave function which determines the motion of a single electron is called orbital, χ(r), and depends on the position vector r. If the one-electron wave function is for an atomic system, it is called an atomic orbital (AO).
The one-electron wave function, χ(r), is interpreted as a “probability amplitude” for the electron. The square modulus of the wave function is interpreted as the “probability density” to find an electron:
| ρ(r) = |χ(r)|2 | (1) |
Quantum mechanics only tells us the probability for some quantum events to occur. The probability to find an electron over all the three-dimensional space is equal to 1, and it is given by the integral of the probability density over ℝ3
| ∫ ℝ3 ρ(r) d3r = +∞ ∫ 0 |χ(r)|2 d3r = 1 | (2) |
In other words, the previous equation provides the number of electrons and the electronic charge in ℝ3. The product ρ(r) d3r gives the probability of finding the electron in an infinitesimal volume element d3r (or dV). If the components of r are the cartesian coordinates x, y, and z, then the volume element is dV = dx dy dz.
Though most atoms, when they are free, are not spherical, still it may be useful to think of an atom as some sort of fuzzy ball of electrons around a small, central, heavy nucleus. And it easy to extend such a mental picture even to bonded atoms in molecules and crystals. For this reason, it is more convenient to express the electron density in spherical coordinates, r, θ and φ, which are related to the cartesian coordinates x, y and z by the transformation
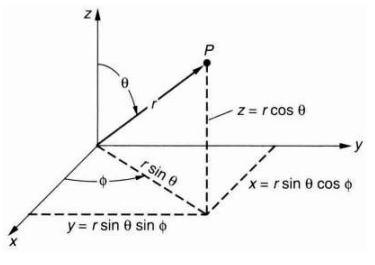
|
|
The expression of the density function under the transformation of the
random vector variable r is discussed in
Section 4.7 of
the chapter on "Charge Distributions"“Transformation of the Random
Vector Variable”
PAMoC Manual: Charge Distributions, Section
4.7, Equations (4.7.4) and (4.7.10).. The conclusion is that,
passing from the reference system of Cartesian axes x, y and
z to the reference system of curvilinear coordinates r,
θ and φ, the value of the probability density must be
multiplied by the determinant of the Jacobian matrix of the transformation of
coordinates, which in the case of spherical coordinates is r2 sin θ:
|
ρ(r,θ,φ) =
ρ(x,y,z) det
|
∂ r
∂
r
∂ r
∂
θ
∂ r
∂
φ
|
= ρ(x,y,z) det
= ρ(x,y,z) r2 sinθ | (4) |
In general an orbital has the structure
| χn,ℓ,m(r) = Rn(r) Yℓ,m(θ,φ) | (5) |
where Rn(r) are radial functions and
Yℓ,m(θ,φ) are spherical
harmonics [L1(a) Wikipedia contributors,
"Spherical harmonics,"
Wikipedia, The Free Encyclopedia.
(b)
Citizendium contributors, "Spherical harmonics."
Citizendium,
The Citizens' Compendium.
(c) Weisstein, E. W. "Spherical
Harmonic,"
From MathWorld - A Wolfram Web Resource.,
L2Wikipedia contributors, "Table of spherical
harmonics,"
Wikipedia, The Free Encyclopedia., N1Real Spherical Harmonics.] of degree ℓ
and order m. Spherical harmonics can be represented using spherical
coordinates (which are convenient when computing integrals) or polynomials
(as is commonly done when evaluating them).
The integer n = 1, 2, … is the principal quantum number, the
integer ℓ = 0, 1, … n − 1 is the azimuthal
quantum number, and the integer m = −ℓ, −ℓ
+ 1, …, ℓ − 1, ℓ is the magnetic quantum number.
By virtue of eq. (4) combined with eq. (5), the electron density function
in the system of curvilinear coordinates r, θ and
φ is given by
|
ρn,ℓ,m(r,θ,φ) =
| χn,ℓ,m(r,θ,φ)
|2 r2 sinθ
= [ r2 |Rn(r)|2 ] [ sinθ |Yℓ,m(θ,φ)|2 ] = ρn(r) ρℓ,m(θ,φ) | (6) |
This is the joint probability
density“Joint and Marginal Probability Density Function”
PAMoC User's Manual which is related to the probability
ρn,ℓ,m(r,θ,φ) dr dθ dφ of finding the electron in an
infinitesimal volume element, which in spherical coordinatess, is given by
dV = r2 sinθ
dr dθ dφ.[N2Volume element in spherical coordinates.]
The joint probability density is factorized into the product of two
marginal densities,
ρn(r) and
ρℓ,m(θ,φ) .
The marginal density
function“Joint and Marginal Probability Density Function”
PAMoC User's Manual
ρℓ,m(θ,φ) is defined by
ρℓ,m(θ,φ) =
|Yℓ,m(θ,φ)|2
sinθ
| (7) |
and is related to the probability ρℓ,m(θ,φ) dθ dφ of finding the electron on a surface element of the unit sphere,[N3Surface area element in spherical coordinates.] i.e. the element of solid angle dΩ = sinθ dθ dφ.[N4Solid angle element in spherical coordinates.]
The radial electron
density“Joint and Marginal Probability Density
Function”
PAMoC User's Manual
ρn(r), also referred to as the radial
distribution, is defined by
ρn(r) =
r2 |Rn(r)|2
| (8) |
and is related to the probability ρn(r) dr that a measurement of the electron's position yields a value anywhere in a spherical shell of infinitesimal thickness at a distance r from the nucleus as shown in the following figure for the radial distribution with n = 1.[N5Volume of a spherical shell of infinitesimal thickness.]
What the radial probability distribution shows is that the electron cannot
be sucked into the nucleus because ρn(0) = 0.
Hence, as we shrink the radial shell into the nucleus, the probability of
finding the electron in that shell goes to 0.[L3Mark E. Tuckerman
“The Schrödinger equation
for the hydrogen atom and hydrogen-like cations”
2011-10-26.]
The density functions in eqs (7-9) are normalized to 1, meaning that the probability to find the electron in ℝ3 as well as the number of electrons and the electronic charge in ℝ3 are equal to 1.
| +∞ ∫ r=0 π ∫ θ=0 2π ∫ φ=0 ρn,ℓ,m(r,θ,φ) dr dθ dφ = +∞ ∫ r=0 π ∫ θ=0 2π ∫ φ=0 | χn,ℓ,m(r,θ,φ) |2 r2 sinθ dr dθ dφ = 1 | (9) |
| π ∫ θ=0 2π ∫ φ=0 ρℓ,m(θ,φ) dθ dφ = π ∫ θ=0 2π ∫ φ=0 |Yℓ,m(θ,φ)|2 sinθ dθ dφ = 1 | (10) |
| +∞ ∫ r=0 ρn(r) dr = +∞ ∫ r=0 r2 |Rn(r)|2 dr = 1 | (11) |
The normalization property of the radial density function, eq. (11), is strictly related to the radial cumulative distribution function Dn(r), which is defined as the probability to find the electron in the interval [0,r] and has the following expression
| Dn(r) = r ∫ 0 ρn(r') dr' = r ∫ 0 r'2 |Rn(r')|2 dr' | (12) |
(see section 4.2 of the chapter on “Charge Distributions” for more details). It satisfies the relationships D(0) = 0, limr→+∞ D(r) = 1, and dD(r) dr = ρ(r). In addition, the probability to find the electron in any interval [a,b] (∀ 0 ≤ a < b ∈ ℝ) is given by the difference D(b) − D(a). The joint cumulative distribution functions Dn,ℓ,m(r,θ,φ) and Dℓ,m(θ,φ) are defined in a similar way.
Explicit expressions for normalized Slater-type orbitals (STO) and
Gaussian-type orbitals (GTO) in spherical coordinates and in Cartesian
coordinates are given in reference [1Gomes,
A. S. P.; Custodio, R.
J. Comput. Chem. 2002, 23,
1007-1012.].
| Spherical STO | Spherical GTO | ||
| Orbital | χn,ℓ,m(ζ; r,θ,φ) = Rn(ζ; r) Yℓ,m(θ,φ) | χn,ℓ,m(α; r,θ,φ) = Rn(α; r) Yℓ,m(θ,φ) | (D1) |
| Radial function | Rn(ζ; r) = Nn(ζ) rn−1 e−ζr | Rn(α; r) = Nn(α) rn−1 e−αr2 | (D2) |
| Normalizing constant |
Nn(ζ) = [ (2ζ)2n+1 (2n)! ]1⁄2 | Nn(α) = [ 24n+3 α2n+1 [(2n − 1)!!]2 π ]1⁄4 | (D3) |
| Cartesian STO | Cartesian GTO | ||
| Orbital | χa,b,c,k(ζ; x,y,z) = Na,b,c,k(ζ) xa yb zc rk−1 e−ζr | χa,b,c(α; x,y,z) = Na,b,c(α) xa yb zc e−αr2 | (D4) |
|
Normalizing constant |
Na,b,c,k(ζ) = [ (2ζ)2k+1 (2k)! (2a + 2b + 2c + 1)!! 4π (2a − 1)!! (2b − 1)!! (2c − 1)!! ]1⁄2 | Na,b,c(α) = [ 22(a + b + c) + 3⁄2 αa + b + c + 3⁄2 π3⁄2 (2a − 1)!! (2b − 1)!! (2c − 1)!! ]1⁄2 | (D5) |
where n, ℓ, and m are the well-known quantum
numbers, in addition k = n − (a + b +
c) and a, b, and c are integers.
The sum a + b + c = ℓ is usually referred
to as s, p, d, etc., even though they generally contain components of lower
angular momentum.
The functions Yℓ,m(θ,φ) are
orthonormalized real spherical harmonics. Their expressions can be found in
reference [L2Wikipedia contributors, "Table of
spherical harmonics,"
Wikipedia, The Free Encyclopedia.].
Derivation of the normalization constants can be found on the tutorial pages of this manual (Exercise 1, Exercise 2, Exercise 3, Exercise 4, Exercise 5).
A simple case: the ground state of the hydrogen atom
The Schrödinger equation (in atomic units) for the
hydrogen atom is[2Szabo, A.; Ostlund, N. S.
“Modern Quantum Chemistry - Introduction to Advanced
Electronic Structure Theory”
Macmillan Publishing Co., Inc.,
New York, 1982, p. 33.]
| (− 1 2 ∇2 − Z r ) ❘Φ⟩ = E ❘Φ⟩ | (13) |
where Z is the atomic number and is equal to the nuclear charge. The same equation also applies to hydrogen-like (or hydrogenic) ions, which are constituted by any atomic nucleus with a single electron, so that they are isoelectronic with hydrogen. These ions carry the positive charge (Z − 1) e, where Z is the atomic number of the atom. Left multiplying both sides of eq. (13) by ⟨Φ❘, yields the formal solution
| E1s = ⟨ Φ❘− 1 2 ∇2 − Z r ❘Φ ⟩ ⟨Φ❘Φ⟩ | (14) |
If we use the 1s-STO ❘Φ⟩ ≡ χ1,0,0(r) = R1(r) Y0,0(θ,φ) as a trial function for the ground state energy of the hydrogen atom, eq. (14) can be rewritten as
| E1s = ⟨ R1(r)❘− 1 2 ∇2 − Z r ❘R1(r) ⟩ ⟨ Y0,0(θ,φ) ❘ Y0,0(θ,φ) ⟩ ⟨Φ❘Φ⟩ | (15) |
Both the radial part, R1(r), and the angular part, Y0,0(θ,φ), of the 1s-STO χ1,0,0(r) as well as the 1s-STO itself are normalized separately, i.e.
|
⟨Y0,0(θ,φ)❘Y0,0(θ,φ)⟩ =
1
4 π
π
∫
0
sinθ dθ
2π
∫
0
dφ
= 1 4 π ⋅ [−cosθ ]π0 ⋅ [φ ]2π0 = 1 4 π ⋅ 2 ⋅ 2 π = 1 | (16) |
where the spherical harmonic of degree ℓ = 0 and order
m = 0 is the constant
Y00 =
1
2
√
1
π
,
[L2Wikipedia contributors, "Table of spherical
harmonics,"
Wikipedia, The Free Encyclopedia.]
| ⟨R1(r)❘R1(r)⟩ = 4 ζ3 +∞ ∫ 0 e−2ζ r r2 dr = 4 ζ3 ⋅ 1 4 ζ3 = 1 | (17) |
where the radial part of the 1s-STO has the expression R1(r) = 2 √ζ3 e−ζ r (cf the table in the “details” section above), and
| ⟨Φ❘Φ⟩ ≡ ⟨χ1,0,0(r)❘χ1,0,0(r)⟩ = ⟨R1(r)❘R1(r)⟩ ⟨Y0,0(θ,φ)❘Y0,0(θ,φ)⟩ = 1 | (18) |
where the 1s-STO has the espression χ1,0,0(r) = √ ζ3 π e−ζ r.
Substituting the Laplace operator ∇2 in eq. (15) with its expression in spherical coordinates, and remembering the normalization conditions (16-18), yields
|
E1s =
⟨
R1(r)❘−
1
2
d2
d
r2 −
1
r
d
d
r
−
Z
r
❘R1(r)
⟩
= ⟨ R1(r)❘− ζ2 2 − 1 r (Z − ζ) ❘R1(r) ⟩ = − ζ2 2 ⟨R1(r)❘R1(r)⟩ + (ζ − Z) ⟨ R1(r)❘ 1 r ❘R1(r) ⟩ = − ζ2 2 + 4 ζ3 (ζ − Z) +∞ ∫ 0 r e−2ζ r dr = − ζ2 2 + 4 ζ3 (ζ − Z) 1 (2 ζ)2 = − ζ2 2 + ζ (ζ − Z) | (19) |
In deriving eqs (17) and (19) use of the integral
+∞
∫
0
rn e−γ r
dr =
n!
γn+1
has been made [3“A
Concise Handbook of Mathematics, Physics, and Engineering Sciences”
Polyanin, A. D., Chernoutsan, A. I., Eds.
Taylor & Francis Group: New York, 2011.] (cf the
Exercise 2 to see how the integral can be
worked out). The optimal value of the Slater exponent ζ is
determined by calculating the energy derivative with respect to ζ
and equating to zero, i.e.
| d E1s d ζ = ζ − Z = 0 ⇒ ζ = Z |
so that eq. (19) yields
| E1s = − Z2 2 | (20) |
The radial density function of the ground state of the hydrogen atom is given by eq.(8) with n = 1, i.e.
ρ1s(r) = r2
|R(r)|2 =
4 ζ3 r2
e− 2 ζ r
| (21) |
STOs with n ≥ 1 differ significantly from the exact solutions of
eq. (13) for hydrogenic atoms.[4Leonard I.
Schiff
“Quantum Mechanics”
McGraw-Hill,
1968, Chapter 16, pp. 88-99.,
5Paul A. Tipler and Gene Mosca
“Physics for Scientists and Engineers: Extended Version”.
W. H. Freeman, 2003, Section 36-4, p. 1181.,
6David A. B. Miller
“Quantum
Mechanics for Scientists and Engineers”
Cambridge University
Press, 2008, Chapter 10, pp. 259-278.,
L6Citizendium contributors
“Hydrogen-like atom”
Citizendium, The Citizens'
Compendium.]
The largest difference is the absence of nodes in the radial dependence of
the wavefunction.
| ψn,ℓ,m(α; r,θ,φ) = Rn,ℓ(α; r) Yℓ,m(θ,φ) |
Wikipedia, The Free Encyclopedia.
(b) Citizendium contributors, "Spherical harmonics."
Citizendium, The Citizens' Compendium.
(c) Weisstein, E. W. "Spherical Harmonic,"
From MathWorld - A Wolfram Web Resource., L2Wikipedia contributors, "Table of spherical harmonics,"
Wikipedia, The Free Encyclopedia., N1Real Spherical Harmonics.] of degree ℓ and order m. The functions Rn,ℓ(α; r) describe the radial dependence of the wavefunction
| Rn,ℓ(α; r) = Nn,ℓ(α) ( 2Z r n ) ℓ L2ℓ+1n−ℓ−1 ( 2Z r n ) exp ( − Z r n ) |
| Nn,ℓ(α) = √ (n − ℓ − 1)! 2n (n + ℓ)! ( 2Z n ) 3 |
| χn,ℓ,m(ζ; r,θ,φ) = Rn(ζ; r) Yℓ,m(θ,φ) |
| Rn(ζ; r) = Nn(ζ) rn−1 e−ζ r |
| R(s) = sℓ L(s) exp(−s/2) |
“Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables”.
New York: Dover, 1972, § 22.3, p. 775.] are defined as
| Ljp(s) = p ∑ q=0 (−1)q ( p + j p − q ) 1 q! sq = p ∑ q=0 (−1)q (p + j)! (p − q)! (j + q)! q! sq |
| [Nn,ℓ]2 +∞ ∫ 0 [Rn,ℓ(r)]2 r2 dr ≡ [Nn,ℓ]2 ( n 2Z ) 3 +∞ ∫ 0 [Rn,ℓ(s)]2 s2 ds = 1 |
| +∞ ∫ 0 [Rn,ℓ(s)]2 s2 ds = +∞ ∫ 0 s2ℓ [L2ℓ+1n−ℓ−1(s) ]2 e−s s2 ds = 2n (n + ℓ)! (n − ℓ − 1)! |
| Nnℓ = √ (n − ℓ − 1)! 2n (n + ℓ)! ( 2Z n ) 3 |
(d) ζ = 2n − 1 n Z.
Rnℓ(r) = √ (n − ℓ − 1)! 2n (n + ℓ)! ( 2Z n ) 3 ( 2Z r n ) ℓ L2ℓ+1n−ℓ−1 ( 2Z r n ) exp ( − Z r n )
Rn(r) = √ (2ζ)2n+1 (2n)! rn−1 e−ζ r
Exercise: Find the moment generating function of an exponential random variable r with parameter ζ, e.g. the radial density function of the gound state of the hydrogen atom .
| Mr(s) = E(es r) = +∞ ∫ 0 es r ρ(r) dr = γ3 2 +∞ ∫ 0 e−(γ−s)r r2 dr = γ3 (γ − s)3 |
| dn d sn Mr(s) = dn d sn [γ (γ − s)−1]3 = n! 2 (γ − s)n |
| dn d sn Mr(s)❘s=0 = n! 2 γn. |
| ⟨rn⟩ ≡ E(rn) = +∞ ∫ 0 rn ρ(r) dr = γ3 2 +∞ ∫ 0 e−γ r rn+2 dr = n! 2 γn. |
Exercise: Use an 1s-GTO, as a trial function, to find an upper bound to the exact ground state energy of the hydrogen atom .
| ❘Φ⟩ = ( 2α π )3⁄4 e−α r2 | (t) |
|
⟨Φ❘Φ⟩ =
(
2α
π
)3⁄2
+∞
∫
0
e−2α r2
r2 dr
π
∫
0
sinθ dθ
2π
∫
0
dφ
= ( 2α π )3⁄2 ⋅ π½ 4 (2 α)3/2 ⋅ 4 π = 1 | (u) |
|
E =
⟨
Φ❘−
1
2
d2
d
r2 −
1
r
d
d
r
−
Z
r
❘Φ
⟩
=
⟨
Φ❘− 2
α2 r2 + 3 α −
Z
r
❘Φ
⟩
= 4 π ( 2α π )3⁄2 ( − 2 α2 +∞ ∫ 0 e−2α r2 r4 dr + 3 α +∞ ∫ 0 e−2α r2 r2 dr − Z +∞ ∫ 0 r e−2α r2 dr ) = 4 π ( 2α π )3⁄2 [ 3 16 ( π 2 α )1⁄2 − Z 4 α ] = 1 2 ( 3 α − 8 Z √ α 2 π ) | (v) |
Polyanin, A. D., Chernoutsan, A. I., Eds.
Taylor & Francis Group: New York, 2011.] +∞ ∫ 0 r2n e−γ r2 dr = (2 n − 1)!! 2n+1 γn ( π γ )1⁄2 = (2 n)! 22n+1 n! γn ( π γ )1⁄2 and +∞ ∫ 0 r2n+1 e−γ r2 dr = n! 2 γn+1 , have been used. The optimal value of the exponent α is obtained by solving the equation d E d α = 0, i.e.
| 3 − 4 Z √ 2 π α = 0 ⇒ α = 8 Z2 9 π = 0.28294246 Z2 | (w) |
“Modern Quantum Chemistry - Introduction to Advanced Electronic Structure Theory”
Macmillan Publishing Co., Inc., New York, 1982, p. 33.] Finally, the normalization constant becomes
| ( 2α π )3⁄4 = 8 ( Z 3 π )3⁄2 = 0.27649271 Z3/2 | (x) |
|
ρ1s(r) =
64
27
(
Z
π
)3
r2 exp
(
−
16 Z2
9 π
r2
)
| (y) |
Exercise: Write the wave function files (.wfn) for the hydrogen atom using both Slater and Gaussian basis sets.
Name H Run Type SinglePoint Method ROHF Basis Set Slater
SLATER 1 MOL ORBITALS 1 PRIMITIVES 1 NUCLEI
H (CENTRE 1) 0.00000000 0.00000000 0.00000000 CHARGE = 1.0
CENTRE ASSIGNMENTS 1
TYPE ASSIGNMENTS 1
EXPONENTS 1.0000000E+00
MO 1 OCC NO = 1.00000000 ORB. ENERGY = -0.5
0.56418958E+00
END DATA
THE SCF ENERGY = -0.500000000000 THE VIRIAL(-V/T)= 2.00000000
2) Gaussian basis set (a single 1s orbital, according to eq.D?):
Name H Run Type SinglePoint Method ROHF Basis Set STO-1G
GAUSSIAN 1 MOL ORBITALS 1 PRIMITIVES 1 NUCLEI
H (CENTRE 1) 0.00000000 0.00000000 0.00000000 CHARGE = 1.0
CENTRE ASSIGNMENTS 1
TYPE ASSIGNMENTS 1
EXPONENTS 0.2829425E+00
MO 1 OCC NO = 1.00000000 ORB. ENERGY = -0.42441369
0.27649271E+00
END DATA
THE SCF ENERGY = -0.424413690000 THE VIRIAL(-V/T)= 2.00000000
References
- “Exact Gaussian Expansions of Slater-Type Atomic
Orbitals”
Gomes, A. S. P.; Custodio, R. J. Comput. Chem. 2002, 23, 1007-1012. - Szabo, A.; Ostlund, N. S. “Modern Quantum Chemistry − Introduction to Advanced Electronic Structure Theory” Macmillan Publishing Co., Inc., New York, 1982, p. 33. ISBN 0-02-949710-8.
- “A Concise Handbook of Mathematics, Physics, and Engineering Sciences”; Polyanin, A. D., Chernoutsan, A. I., Eds.; Taylor & Francis Group: New York, 2011.
- Leonard I. Schiff, “Quantum Mechanics”. McGraw-Hill, 1968, Chapter 16, pp. 88-99. ISBN-13: 9780070856431. ISBN-10: 0070856435.
- Paul A. Tipler; Gene Mosca; “Physics for Scientists and Engineers: Extended Version”. W. H. Freeman, 2003, Section 36-4, p. 1181. ISBN-13: 9780716743897. ISBN-10: 0716743892.
- David A. B. Miller, “Quantum Mechanics for Scientists and Engineers”. Cambridge University Press, 2008, Chapter 10, pp. 259-278. ISBN-13: 9780521897839. ISBN-10: 0521897831.
- Milton Abramowitz and Irene A. Stegun, eds. “Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables”. United States Government Printing Office, 1972, § 22.3, p. 775. ISBN-13: 9780318117300. ISBN-10: 0318117304.
- A. V. Arecchi, T. Messadi, and R. J. Koshel, “Field Guide to Illumination”, SPIE Press, Bellingham, WA, 2007, p. 2. ISBN: 9780819467683.
Notes
- Real Spherical Harmonics. Orthonormalized Laplace’s
spherical harmonics are defined as
where Pℓm(cosθ) is the associate Legendre polynomial of degree ℓ and order m ( ℓ ≥ 0 and −ℓ≤ m ≤ ℓ). Spherical harmonics are single-valued, smooth (infinitely differentiable), complex functions of two variables, θ and φ, indexed by two integers, ℓ and m, and form a complete set of orthonormal functions:Yℓm(θ,φ) = (−1)m Nℓm Pℓm(cosθ) eimφ (N1.1)
This normalization is used in quantum mechanics because it ensures that probability is normalized, i.e. π ∫ 0 |Yℓm(Ω)|2 dΩ = 1, with dΩ = sinθ dθ and normalization factor:2π ∫ 0 dφ π ∫ 0 Yℓm(θ,φ) Yℓ'm'*(θ,φ) sinθ dθ = δℓℓ' δmm' (N1.2)
The real forms of the spherical harmonics, also known as tesseral harmonics, are obtained by taking the linear combinations [Yℓm(θ,φ) ± Yℓ−m(θ,φ)]/√2. They are defined as followsNℓm = √ 2ℓ + 1 4 π ⋅ (ℓ − |m|)! (ℓ + |m|)! (N1.3)
whereYℓm(θ,φ) = Mℓm Uℓm(θ,φ) (N1.4)
are the un-normalized tesseral harmonics, and Mℓm is the normalization factorUℓm(θ,φ) = Pℓ|m|(cosθ) { cos(|m|φ), m ≥ 0 sin(|m|φ), m < 0
(N1.5)Mℓm = Nℓm √ 2 − δ|m|,0 = √ 2ℓ + 1 4 π ⋅ (2 − δ|m|,0) ⋅ (ℓ − |m|)! (ℓ + |m|)! (N1.6) - Volume element in spherical coordinates.
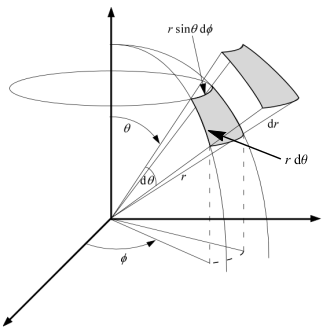
Working in spherical coordinates, the diagram on the left enable us to calculate the infinitesimal volume element as
The volume of a solid is always given by the integration of the infinitesimal volume element dV over the entire solid. We cover a sphere of radius R if we let r ∈ [0,R], θ ∈ [0,π], and φ ∈ [0,2π]. The volume of the sphere is then given bydV = dr ⋅ r dθ ⋅ r sinθ dφ = r2 sinθ dr dθ dφ V = ∫ sphere dV = R ∫ 0 r2 dr π ∫ 0 sinθ dθ 2π ∫ 0 dφ
= [ 1 3 r3 ]R0 ⋅ [−cosθ ]π0 ⋅ [φ ]2π0
= 4 π R3 3 - Surface area element in spherical coordinates.
The diagram of the previous note [N2] also enables us
to calculate the infinitesimal area elements when we integrate over only
two of the spherical coordinates. When we integrate over the surface of
a sphere, we only vary θ and φ by dθ
and dφ, respectively, and we do not vary r at all. The
corresponding area element is given in the diagram of the previous note as
The surface area of a sphere of radius R is obtained by direct integration over the entire sphere, letting θ ∈ [0,π] and φ ∈ [0,2π] while using the spherical coordinate area element R2 sinθ dθ dφ,dA = r2 sinθ dθ dφ A = ∫ sphere dA = R2 π ∫ 0 sinθ dθ 2π ∫ 0 dφ
= R2 ⋅ [−cosθ ]π0 ⋅ [φ ]2π0
= 4 π R2 - Solid angle. A solid angle is the 3 dimensional analog of
an ordinary angle. It is related to the surface area of a sphere in the same
way an ordinary angle is related to the circumference of a circle.
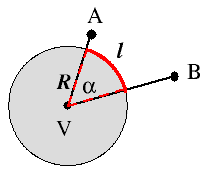
A plane angle, α, is made up of the lines that from two points A and B meet at a vertex V: it is defined by the arc length (red curve in the figure on the left) of a circle subtended by the lines and by the radius R of that circle (dotted red lines),[8A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2., L4"Solid Angle". Optipedia: SPIE Press books opened for your reference.
Excerpt from "Field Guide to Illumination" by Angelo V. Arecchi, Tahar Messadi, R. John Koshel.] as shown on the left. The dimensionless unit of plane angle is the radian. The value in radians of a plane angle α is the ratio
of the length ℓ of a circular arc to its radius R. It follows that the plane angle subtended by the full circle measures 2π radians.α = ℓ R
The infinitesimal element of a solid angle Ω is given by dΩ = dA R2 , where dA is the surface area element in spherical coordinates defined in [N3Surface area element in spherical coordinates.], so thatA solid angle is made up from all the lines that from a closed curve meet at a vertex: it is defined by the surface area A of a sphere subtended by the lines and by the radius R of that sphere,[8A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2., L4"Solid Angle". Optipedia: SPIE Press books opened for your reference.
Excerpt from "Field Guide to Illumination" by Angelo V. Arecchi,Tahar Messadi, R. John Koshel.] as shown in the Figure on the right. The dimensionless unit of solid angle is the steradian. The measure in steradians of a solid angle Ω is the area A on the surface of a sphere of radius R divided by the radius squared,
and is independent of the particular value of the chosen radius. Because the area A of the entire spherical surface is 4πr2, the definition implies that a sphere subtends 4π steradians at its center. By the same argument, the maximum solid angle that can be subtended at any point is 4π steradians.Ω = A R2 ,
Concisely, the solid angle Ω subtended by a surface S is defined as the surface area Ω of a unit sphere covered by the surface’s projection onto the sphere.[L5(a) Weisstein, E. W. "Solid Angle,"
From MathWorld - A Wolfram Web Resource.
(b) Wikipedia contributors, "Solid angle,"
Wikipedia, The Free Encyclopedia.] href="#L4">L4Weisstein, E. W. "Solid Angle,"
From MathWorld - A Wolfram Web Resource.] That’s a very complicated way of saying the following: You take a surface, like the bright red circle in the figure on the right. Then you project the edge of the circle (but in general it could be any closed curve) to the center of a sphere of any radius R. The projection intersects the sphere and forms a surface area A. Then you calculate the surface area of your projection and divide the result by the radius squared. That's it.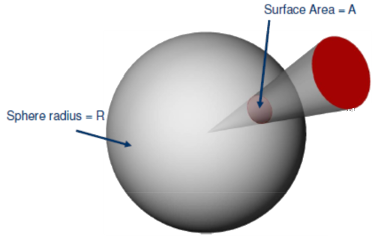
is equal to the differential surface area on the unit sphere. Its integral over the solid angle of all space being subtended is the surface area of the unit sphere, which is 4 π steradians.[L5(a) Weisstein, E. W. "Solid Angle,"dΩ = sinθ dθ dφ
From MathWorld - A Wolfram Web Resource.
(b) Wikipedia contributors, "Solid angle,"
Wikipedia, The Free Encyclopedia.]Ω = ∫ Ω dΩ = π ∫ 0 sinθ dθ 2π ∫ 0 dφ = [−cosθ ]π0 ⋅ [φ ]2π0 = 4 π - Volume of a spherical shell of infinitesimal thickness.
Consider two spheres, both centered in the origin: the inner with radius
r and the outer with radius r + dr. To compute the
volume of the spherical shell between their two surfaces, proceed as follows:
where, in the last equality, the terms drn with n > 1 have been neglected. In a different way, using differentiation of volume V = 4 3 π r3 w.r.t. radius, you get area (rate of change of volume is area)dV = 4 3 π (r + dr)3 − 4 3 π r3 = 4 3 π (r3 + 3r2dr + 3r dr2 + dr3 − r3 (N5.1) = 4 3 π (3r2dr + 3r dr2 + dr3) ≈ 4 π r2dr d V d r = 4 π r2 ⇒ d V = 4 π r2 d r (N5.2)
Links
- (a) Wikipedia contributors, "Spherical harmonics."
Wikipedia, The Free Encyclopedia.
https://en.wikipedia.org/wiki/Spherical_harmonics (accessed October 3, 2018).
(b) Citizendium contributors, "Spherical harmonics." Citizendium, The Citizens' Compendium.
http://en.citizendium.org/wiki/Spherical_harmonics (accessed January 23, 2019).
(c) Weisstein, Eric W. “Spherical Harmonic.” From MathWorld − A Wolfram Web Resource.
http://mathworld.wolfram.com/SphericalHarmonic.html (accessed October 3, 2018). - Wikipedia contributors, "Table of spherical harmonics." Wikipedia,
The Free Encyclopedia.
https://en.wikipedia.org/wiki/Table_of_spherical_harmonics (accessed October 3, 2018). - Mark E. Tuckerman, “The Schrödinger equation for the
hydrogen atom and hydrogen-like cations”, 2011-10-26.
(Lecture 8: CHEM-UA 127: Advanced General Chemistry I)
http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_8/node5.html (accessed October 31, 2018). - “Solid Angle”. Optipedia: SPIE Press books opened for
your reference. Excerpt from “Field Guide to Illumination” by
Angelo V. Arecchi, Tahar Messadi, R. John Koshel.[4A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2.]
http://spie.org/publications/fg11_p02_solid_angle (accessed November 12, 2018). - (a) Weisstein, Eric W. “Solid Angle.”
From MathWorld − A Wolfram Web Resource.
http://mathworld.wolfram.com/SolidAngle.html (accessed October 4, 2018).
(a) Wikipedia contributors, “Solid angle.” Wikipedia, The Free Encyclopedia.
https://en.wikipedia.org/wiki/Solid_angle (accessed October 4, 2018). - Citizendium contributors, “Hydrogen-like atom”;
Citizendium, The Citizens' Compendium.
http://en.citizendium.org/wiki/Hydrogen-like_atom (unapproved; accessed December 17, 2018).