Exercises
- Evaluate the integral I(0) = +∞ ∫ 0 e−γ rk dr.
- Evaluate the integral I(n) = +∞ ∫ 0 rn e−γ rk dr.
- Evaluate the normalization constant of a Slater-type orbital (STO) and a Gaussian-type orbital (GTO) in spherical coordinates.
- Evaluate the normalization constant of a Gaussian-type orbital (GTO) in Cartesian coordinates.
- Evaluate the normalization constant of a Slater-type orbital (STO) in Cartesian coordinates.
- Use the variation method to show that the orbital energies obtained by solving the Schrödinger equation for hydrogenic atoms with the Slater-type orbital χn,ℓ,m(ζ; r,θ,φ) = Rn(ζ; r) Yℓ,m(θ,φ) as a trial function are an upper bound to the exact orbital energies, En = − Z2 2 n2 . Compare the behavior of Slater-type orbitals against that of exact hydrogenic orbitals, and find the relationship between hydrogenic and Slater-type orbitals.
- Find the average distance of the electron from the nucleus in each state of the hydrogen atom.
- Compare the most probable distances of the electron from the proton in the hydrogen 1s, 2s and 2p states with the corresponding average radius.
By using the method of u-substitution, [N5] let u = γ rk so that r = ( u γ )1⁄k and dr = (γ)−1⁄k (u)(1⁄k)−1 k du. Now substitute into the integrand, replacing all forms of r:
| I(0) = (γ)−1⁄k k −∞ ∫ 0 (u)(1⁄k)−1 e−u du = (γ)−1⁄k k Γ ( 1 k ) = (γ)−1⁄k Γ (1 + 1 k ) | (E1.1) |
The improper integral in eq. (E1.1) is easily recognized as an Euler integral of the second kind[L1Euler integrals. Encyclopedia of Mathematics.], i.e. the gamma function Γ(1⁄k).[L2Wikipedia contributors, "Gamma function," Wikipedia, The Free Encyclopedia., L3Weisstein, Eric W. "Gamma Function." From MathWorld--A Wolfram Web Resource.]
Case k = 1: 1s-STO. For positive integers n, the gamma function is related to the factorial function: Γ(n) = (n − 1)!, so that for k = 1 the gamma function is Γ(2) = 1! = 1 and I(0) = 1 γ . The same result can be obtained by direct integration, after u-substitution (i.e. u = − γ r, from which r = − u⁄γ and dr = − 1⁄γ du):
|
I(0) =
+∞
∫
0
e−γ r dr = − 1 γ −∞ ∫ 0 eu du = − 1 γ [eu ]−∞0 = − 1 γ ( lim u → −∞ eu − e0 ) = 1 γ | (E1.2) |
Case k = 2: 1s-GTO. The gamma function evaluated at k = 2 is [L2Wikipedia contributors, "Gamma function," Wikipedia, The Free Encyclopedia., L3Weisstein, Eric W. "Gamma Function." From MathWorld--A Wolfram Web Resource.] Γ ( 1 2 ) = √π and I(0) = 1 2 √ π γ . The value of Γ(z) at z = 1⁄2 can be evaluated using Euler's reflection formula [L4Weisstein, Eric W. "Reflection Relation." From MathWorld − A Wolfram Web Resource.]
| Γ(z) Γ(1 − z) = π sin (π z) | (E1.3) |
The result can be proven by direct integration, after u-substitution (i.e. u2 = γ r2, from which r = √ 1 γ u and dr = √ 1 γ du):
| I(0) = +∞ ∫ 0 e−r2 dr = √ 1 γ +∞ ∫ 0 e−u2 du | (E1.4) |
To derive the value for I(0), the following steps are used. First, the value of I(0) is squared. Second, the squared value is rewritten as a double integral. Third, the double integral is evaluated by transforming to polar coordinates. Fourth, the I(0) is explicitly solved for. The first two steps yield
|
I(0)2 =
1
γ
+∞
∫
0
e−x2 dx ×
+∞
∫
0
e−y2 dy = 1 γ +∞ ∫ 0 +∞ ∫ 0 e−(x2 + y2) dx dy | (E1.5) |
where it has been taken into account that the integration variable u is silent, in the sense that it can be chosen freely and, if necessary, replaced with a symbol more appropriate to the context. The region which defines the first quadrant, is the region of integration for the integral in eq. (E1.5). The bivariate transformation x = r cos θ, y = r sin θ will transform the integral problem from cartesian coordinates, (x, y), to polar coordinates, (r, θ). These new variables will range from 0 ≤ r ≤ ∞ and 0 ≤ θ ≤ π⁄2 for the first quadrant. The Jacobian determinant of the transformation is
det |J| = det
| (E1.6) |
so that the area elements is
| dx dy = r dr dθ | (E1.7) |
Hence, eq. (E1.5) can be written as
|
I(0)2 =
1
γ
+∞
∫
0
+∞
∫
0
e−(x2 + y2) dx
dy =
1
γ
π/2
∫
0
+∞
∫
0
e−r2 r dr
dθ = − 1 2 γ π/2 ∫ 0 dθ −∞ ∫ 0 eu du = − 1 2 γ [θ ]π/20 ⋅ [eu ]−∞0 = − 1 2 γ ( π 2 − 0 ) ( lim u → −∞ eu − e0 ) = − π 4 γ ( 0 − 1 ) = π 4 γ | (E1.8) |
where the substitution u = −r2, du = −2rdr, and dr = − 1 2 r du has been applied to solve the radial integral. Hence,
| I(0) = 1 2 √ π γ | (E1.9) |
as it was anticipated.
Solution. The solution of this exercise is summarized by the following equation
| I(0) = +∞ ∫ 0 e−γ rk dr = (γ)−1⁄k Γ (1 + 1 k ) = { 1 γ for k = 1 (STO), 1 2 √ π γ for k = 2 (GTO), | (E1.10) |
Start from the solution of Exercise 1, which gives the result for n = 0. Then use the Leibniz integral rule (which establishes that a derivative and an integral can be interchanged, allowing differentiation under the integral sign):[L5 Weisstein, Eric W. "Leibniz Integral Rule." From MathWorld − A Wolfram Web Resource.]
| ∂n ∂γn +∞ ∫ 0 e−γ rk dr = +∞ ∫ 0 ∂n ∂γn e−γ rk dr (k = 1, 2) | (E2.1) |
The n-th derivative of I(0) in eq. (E1.10) equals the product of the gamma function Γ (1 + 1 k ) times the n-th derivative of (γ)−1⁄k with respect to γ:
|
I(n) =
∂n
I(0)
∂γn = (−1)n 1 k (1 + 1 k ) (2 + 1 k ) … (n − 1 + 1 k ) (γ)−1⁄k−n Γ (1 + 1 k ) = (−1)n 1⋅(k + 1)⋅(2k + 1)⋅(3k + 1)⋅…⋅[(n−1) k + 1] kn γ (nk+1)/k Γ (1 + 1 k ) = (−1)n [kn − (k − 1)]!(k) kn+1 γ (nk+1)/k Γ ( 1 k ) = (−1)n 1 k γ (nk+1)/k Γ (n + 1 k ) | (E2.2) |
where n!(k) denotes the k-th multifactorial of n [L6Weisstein, Eric W. "Multifactorial." From MathWorld--A Wolfram Web Resource.] and some general properties of the gamma function have been used.[L7Wikipedia contributors, "Particular values of the gamma function," Wikipedia, The Free Encyclopedia.]
On the other hand, the n-th derivative of the integrand in eq. (E1.10) with respect to γ is
| ∂n ∂γn e−γ rk = (−1)n rnk e−γ rk | (E2.3) |
Finally, combining eq (E2.3) and the last equality of eq (E2.2) into eq (E2.1), you get the solution of the exercise:
| +∞ ∫ 0 rnk e−γ rk dr = 1 k γ (nk+1)/k Γ (n + 1 k ) | (E2.4) |
which can be specified for k = 1 (STO):
| +∞ ∫ 0 rn e−γ r dr = 1 γn+1 Γ(n + 1) = n! γn+1 | (E2.5) |
and for k = 2 (GTO):
| +∞ ∫ 0 r2n e−γ r2 dr = 1 2 γ (2n+1)/2 Γ (n + 1 2 ) = (2n − 1)!! 2n+1 √ π γ2n+1 | (E2.6) |
Setting ½n → n in eq (E2.6) you get
| +∞ ∫ 0 rn e−γ r2 dr = 1 2 γ (n+1)/2 Γ ( n + 1 2 ) = (n − 1)!! √ π 2(2γ)n+1 | (E2.7) |
In the eqs (E2.5-7) the identities Γ(n + 1) = n!, Γ (n + 1 2 ) = (2n − 1)!! 2n √π, and Γ ( n + 1 2 ) = (n − 1)!! 2n/2 √π have been used.
Exercise 3: Evaluate the normalization constant of a Slater-type orbital (STO, k = 1) and a Gaussian-type orbital (GTO, k = 2), expressed in spherical coordinates χn,ℓ,m(η,k; r,θ,φ) = Rn(η,k; r) Yℓ,m(θ,φ), where the Yℓ,m(θ,φ) are orthonormalized spherical harmonics, and Rn(η,k; r) = Nn(η,k) rn−1 e−η rk is the radial function, with Nn(η,k) being the normalization constant to be determined.The normalization constant of an atomic orbital χ(r) can be computed by imposing the condition
| ∫ ℝ3 |χ(r)|2 d3r = 1 | (E3.1) |
which, for STOs and GTOs in spherical coordinates, can be written as
| ∫ ℝ3 |Rn(η,k; r) Yℓ,m(θ,φ)|2 dV = +∞ ∫ 0 r2 |Rn(η,k; r)|2 dr π ∫ 0 2π ∫ 0 |Yℓ,m(θ,φ)|2 sinθ dθ dφ = 1 | (E3.2) |
| +∞ ∫ 0 r2 |Rn(η,k; r)|2 dr = Nn(η,k)2 +∞ ∫ 0 r2n e−2η rk dr = 1 | (E3.3) |
From the last identity of the previous equation, you get
| Nn(γ,k) = [ +∞ ∫ 0 r2n e−γ rk dr ]−1⁄2 | (E3.4) |
where γ = 2η. The values of the integral in eq (E3.4) for k = 1 and k = 2 are given by eqs (E2.5) and (E2.6), respectively. Hence
| Nn(γ,k) = { √ γ2n+1 (2n)! for k = 1 (STO), √ 2n+1 (2n − 1)!! ( γ2n+1 π )1⁄4 for k = 2 (GTO), | (E3.5) |
(cf equations (5) and (10) in reference [1Gomes, A. S. P.; Custodio, R.
J. Comput. Chem.
2002, 23, 1007-1012.]).
The normalization constant of an atomic orbital χ(r) is computed by imposing the condition
| ∫ ℝ3 | χa,b,c(α; x,y,z) |2 d3r = 1 | (E4.1) |
Substituting χ(r) with its expression in Cartesian coordinates and observing that r2 = x2 + y2 + z2, the previous equation can be rearranged as follows:
|
+∞
∫
−∞
+∞
∫
−∞
+∞
∫
−∞
| Na,b,c(α)
xa yb
zc
e−α (x2 +y2 +
z2) |2
dx dy dz = = [ Na,b,c(α) ]2 +∞ ∫ −∞x2a e−2α x2 dx +∞ ∫ −∞y2b e−2α y2 dy +∞ ∫ −∞z2c e−2α z2 dz = = [ Na,b,c(α) ]2 Ix Iy Iz = 1 | (E4.2) |
According to eq (E2.6), the integral Ix is given by
| Ix = +∞ ∫ −∞x2a e−2α x2 dx = 2 +∞ ∫ 0x2a e−2α x2 dx = (2a − 1)!! 2a √ π (2α)2a+1 | (E4.3) |
Similar expressions hold for the integrals Iy and Iz, so that eq (E4.2) can be rewritten as
| [ Na,b,c(α) ]2 Ix Iy Iz = [ Na,b,c(α) ]2 (2a − 1)!! (2b − 1)!! (2c − 1)!! 2a+b+c √ π3 (2α)2(a+b+c)+3 = 1 | (E4.4) |
Hence (cf equation (12) in reference [1Gomes, A. S. P.; Custodio, R.
J. Comput. Chem.
2002, 23, 1007-1012.]):
|
Na,b,c(α) =
√
2a+b+c
(2a − 1)!! (2b − 1)!!
(2c − 1)!!
(
(2α)2(a+b+c)+3
π3
)1⁄4
= √ 22(a+b+c)+3⁄2 (2a − 1)!! (2b − 1)!! (2c − 1)!! αa+b+c+3⁄2 π3⁄2 | (E4.5) |
The solution (cf equation (7) in reference [1Gomes, A. S. P.; Custodio, R.
J. Comput. Chem.
2002, 23, 1007-1012.]) is
| Na,b,c,k(ζ) = √ (2ζ)2k+1 (2k)! (2a + 2b + 2c + 1)!! 4π (2a − 1)!! (2b − 1)!! (2c − 1)!! | (E5.1) |
“Modern Quantum Chemistry - Introduction to Advanced Electronic Structure Theory”
Macmillan Publishing Co., Inc., New York, 1982, p. 33.]
| (− 1 2 ∇2 − Z r ) ❘χ⟩ = E ❘χ⟩ |
Compare the behavior of Slater-type orbitals against that of exact hydrogenic orbitals, and find the relationship between hydrogenic and Slater-type orbitals. Left multiplying both sides of the Schrödinger equation by ⟨χ❘, yields the formal solution
| E = ⟨ χ❘ − 1 2 ∇2 − Z r ❘χ ⟩ ⟨χ❘χ⟩ | (E6.1) |
| Rn(ζ; r) = Nn(ζ) rn−1 e−ζ r | (E6.2) |
| Nn(ζ) = √ (2ζ)2n+1 (2n)! | (E6.3) |
|
E =
⟨
Rn(ζ; r)❘−
1
2
∇2 −
Z
r
❘Rn(ζ; r)
⟩
⟨
Yℓ,m(θ,φ)
❘
Yℓ,m(θ,φ)
⟩
⟨χn,ℓ,m(ζ; r,θ,φ)❘χn,ℓ,m(ζ; r,θ,φ)⟩
= ⟨ Rn(ζ; r)❘− 1 2 ∇2 − Z r ❘Rn(ζ; r) ⟩ | (E6.4) |
|
En =
⟨
Rn(r)❘−
1
2
d2
d
r2 −
1
r
d
d
r
−
Z
r
❘Rn(r)
⟩
= ⟨ Rn(r)❘− ζ2 2 + n ζ − Z r − n (n − 1) 2 r2 ❘Rn(r) ⟩ = − ζ2 2 ⟨Rn(r)❘Rn(r)⟩ + (n ζ − Z) ⟨Rn(r)❘r−1❘Rn(r)⟩ − n (n − 1) 2 ⟨Rn(r)❘r−2❘Rn(r)⟩ | (E6.5) |
| ⟨Rn(r)❘Rn(r)⟩ = 1 | (E6.6) |
| ⟨Rn(r)❘r−1❘Rn(r)⟩ = (2ζ)2n+1 (2n)! +∞ ∫ 0 r2n−1 e−2ζ r dr = (2ζ)2n+1 (2n)! ⋅ (2 n − 1)! (2 ζ)2n = ζ n | (E6.7) |
| ⟨Rn(r)❘r−2❘Rn(r)⟩ = (2ζ)2n+1 (2n)! +∞ ∫ 0 r2n−2 e−2ζ r dr = (2ζ)2n+1 (2n)! ⋅ (2 n − 2)! (2 ζ)2n−1 = 2 ζ2 n (2n − 1) | (E6.8) |
|
En =
−
ζ2
2 +
ζ (n ζ − Z)
n −
(n − 1) ζ2
2n − 1
= ζ [n ζ − 2 (2n − 1) Z] 2 n (2n − 1) | (E6.9) |
| d En d ζ = n ζ − (2n − 1) Z n (2n − 1) = 0 ⇒ ζ = 2n − 1 n Z | (E6.10) |
| En = − Z2 2 n2 (2n − 1) | (E6.11) |
Exact hydrogenic wavefunctions and Slater-type
orbitals.
The exact solution of the time-independent Schrödinger equation for
hydrogenic atoms is described in many quantum-mechanical text-books (e.g. [5Leonard I. Schiff
“Quantum
Mechanics”
McGraw-Hill, 1968, Chapter 16,
pp. 88-99.,
6Paul A. Tipler and Gene Mosca
“Physics for Scientists and Engineers: Extended Version”.
W. H. Freeman, 2003, Section 36-4, p. 1181.,
7David A. B. Miller
“Quantum
Mechanics for Scientists and Engineers”
Cambridge University
Press, 2008, Chapter 10, pp. 259-278.]) as well as on the
web (e.g. [L9?Rudi Winter
“Solving
Schrödinger's equation for the hydrogen atom”
Online
resource.]) and is not reported here. We simply mention that
the eigenfunctions can be factored as follows
| ψn,ℓ,m(α; r,θ,φ) = Rn,ℓ(α; r) Yℓ,m(θ,φ) | (E6.12) |
| Rn,ℓ(α; r) = Nn,ℓ(α) (2αr)ℓ L2ℓ+1n−ℓ−1(2αr) e−αr | (E6.13) |
| Nn,ℓ(α) = √ (n − ℓ − 1)! 2n (n + ℓ)! (2 α)3 | (E6.14) |
“Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables”.
New York: Dover, 1972, § 22.3, p. 775.] are defined as
|
L2ℓ+1n−ℓ−1(2αr)
=
n − ℓ − 1
∑
q=0
(−1)q
(
n + ℓ
n − ℓ − q − 1
)
1
q!
(2αr)q
= n − ℓ − 1 ∑ q=0 (−1)q (n + ℓ)! (n − ℓ − q − 1)! (q + 2ℓ + 1)! q! (2αr)q | (E6.15) |
| Rn,ℓ(α; r) = Nn,ℓ(α) n − ℓ − 1 ∑ q=0 (−1)q (n + ℓ)! (n − ℓ − q − 1)! (2ℓ + q + 1)! q! (2αr)ℓ+q e−αr | (E6.16) |
| (2αr)ℓ+q e−αr = (2α)ℓ+q rℓ+q e−αr = (2α)ℓ+q Rℓ+q+1(α; r) Nℓ+q+1(α) | (E6.17) |
| Rn,ℓ(α; r) = n−ℓ−1 ∑ q=0 uℓq(n) Rℓ+q+1(α; r) | (E6.18) |
| ψn,ℓ,m(α; r,θ,φ) = n−ℓ−1 ∑ q=0 uℓq(n) χℓ+q+1,ℓ,m(α; r,θ.φ) | (E6.19) |
|
uℓq(n) =
(−1)q
(n + ℓ)!
(n − ℓ − q − 1)!
(2ℓ + q + 1)! q!
Nn,ℓ(α)
Nℓ+q+1(α)
(2α)ℓ+q = (−1)q [2n (n + ℓ)! (n − ℓ − 1)! (2ℓ + 2q + 2)!]½ 2n (n − ℓ − q − 1)! (2ℓ + q + 1)! q! | (E6.20) |
Explicit expressions of the expansion coefficients uℓq(n) are reported in Table E6.1 for the lowest values of n. It's worth noting the special values u0,0(n) = 1 and un−1,0(n) = 1 for every value of n = 1, 2, ….
| Rnℓ | R1 | R2 | R3 | R4 | uℓq(n),(a) (q = 0, 1, 2, …, n−ℓ−1) |
|---|---|---|---|---|---|
| R10 | 1 | (−1)q [2 (2q + 2)!]½ 2 (− q)! (1 + q)! q! , (q = 0) | |||
| R20 | 1 | −√3 | (−1)q [2 (2q + 2)!]½ 2 (1 − q)! (1 + q)! q! , (q = 0, 1) | ||
| R21 | 1 | (−1)q [6 (2q + 4)!]½ 2 (− q)! (3 + q)! q! , (q = 0) | |||
| R30 | 1 | − 2 √3 | √10 | (−1)q [2 (2q + 2)!]½ (2 − q)! (1 + q)! q! , (q = 0, 1, 2) | |
| R31 | 2 √6 3 | −√5 | (−1)q 2 [(2q + 4)!]½ (1 − q)! (3 + q)! q! , (q = 0, 1) | ||
| R32 | 1 | (−1)q 2 [5 (2q + 6)!]½ (− q)! (5 + q)! q! , (q = 0) | |||
| R40 | 1 | − 3 √3 | 3 √10 | −√35 | (−1)q 3 [2 (2q + 2)!]½ (3 − q)! (1 + q)! q! , (q = 0, 1, 2, 3) |
| R41 | √5 | − 5 √6 2 | √21 | (−1)q [30 (2q + 4)!]½ (2 − q)! (3 + q)! q! , (q = 0, 1, 2) | |
| R42 | 3 √2 2 | −√7 | (−1)q 3 [10 (2q + 6)!]½ (1 − q)! (5 + q)! q! , (q = 0, 1) | ||
| R43 | 1 | (−1)q 3 [70 (2q + 8)!]½ (− q)! (7 + q)! q! , (q = 0) | |||
| (a) Coefficient of Rℓ+q+1(Z⁄n). | |||||
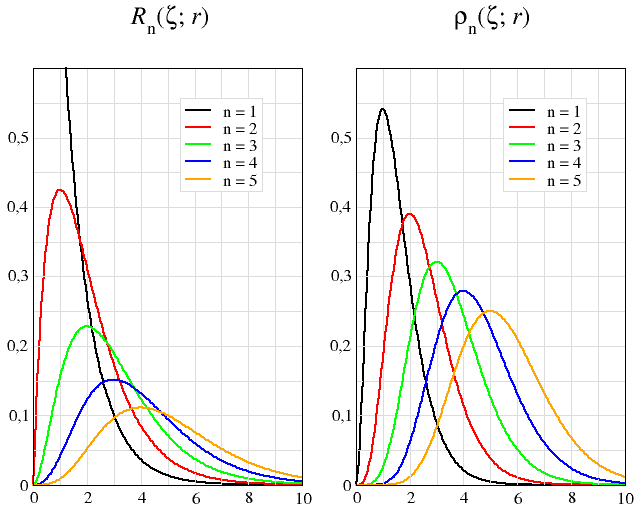
Low order (n = 1, …, 5) hydrogen radial wavefunctions are compared in Table E6.2 with the corresponding Slater-type orbitals with three different exponent values (ζ = α, (2n−1)α, Z). For every value of n, the two functions coincide only if ℓ = n − 1 (in addition to sharing the same exponent, α).
| Rnℓ | exact hydrogenic orbital Rnℓ(α; r) = √ (n − ℓ − 1)! 2n (n + ℓ)! (2 α)3 (2αr)ℓ L2ℓ+1n−ℓ−1(2αr) e−αr α = Z n |
STO approximation Rn(ζ; r) = √ (2ζ)2n+1 (2n)! rn−1 e−ζ r | ||
|---|---|---|---|---|
| ζ = Z n | ζ = (2n − 1) Z n | ζ = Z | ||
| R1,0 ≡ R1s | 2 √Z3 e−Z r | 2 √Z3 e−Z r | 2 √Z3 e−Z r | 2 √Z3 e−Z r |
| R2,0 ≡ R2s | √2 Z3 4 (2 − Z r) e−Z r/2 | √6 Z5 12 r e−Z r/2 | 9 √2 Z5 4 r e−3Z r/2 | 2 √3 Z5 3 r e−Z r |
| R2,1 ≡ R2p | √6 Z5 12 r e−Z r/2 | |||
| R3,0 ≡ R3s | 2 √3 Z3 27 (3 − 2Z r + 2 9 Z2 r2 ) e−Z r/3 | 2 √30 Z7 1215 r2 e−Z r/3 | 250 √6 Z7 243 r2 e−5Z r/3 | 2 √10 Z7 15 r2 e−Z r |
| R3,1 ≡ R3p | √6 Z5 81 r (4 − 2 3 Z r ) e−Z r/3 | |||
| R3,2 ≡ R3d | 2 √30 Z7 1215 r2 e−Z r/3 | |||
| R4,0 ≡ R4s | √Z3 16 (4 − 3Z r + 1 2 Z2 r2 − 1 48 Z3 r3 ) e−Z r/4 | √35 Z9 26880 r3 e−Z r/4 | 2401 √5 Z9 3840 r3 e−7Z r/4 | 2 √35 Z9 105 r3 e−Z r |
| R4,1 ≡ R4p | √15 Z5 192 r (4 − Z r + 1 20 Z2 r2 ) e−Z r/4 | |||
| R4,2 ≡ R4d | √5 Z7 3840 r2 (12 − Z r) e−Z r/4 | |||
| R4,3 ≡ R4f | √35 Z9 26880 r3 e−Z r/4 | |||
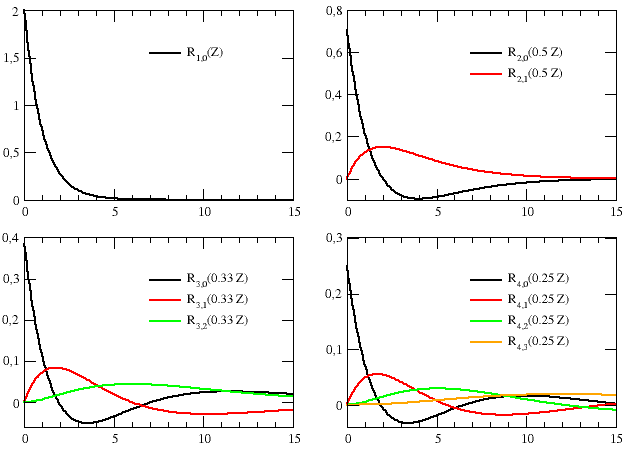
The exercise is completed by the graphs of Figure E6.1, illustrating the behavior of the radial wavefunctions (amplitudes,[N0] R(r)) shown in Table E6.2, and by the graphs of Figures E6.2 and E6.3, which show how the associated squared amplitudes, |R(r)|2, and radial densities, ρ(r) = r2 |R(r)|2, vary as the radius increases.
The radial functions are computed over about 200 points, whose coordinates along a line are expressed in atomic units of length. An ad hoc fortran program provides a human-readable block-data file for each value of n. In each file, the data are structured in (n + 4) columns, the first of which contains the values of the radial coordinate, r. The next n columns contain the values of the hydrogen radial eigenfunctions for ℓ = 0, 1, 2, …, n−1. The last three columns contain the STO values for ζ = Z n , ζ = (2n−1) Z n , and ζ = Z, respectively. C ------------------------------------------------------------------ Program Hydrogen C ------------------------------------------------------------------ C C Compare the radial functions of hydrogenic and Slater-type C orbitals C C ------------------------------------------------------------------ Implicit Double Precision (a-h,o-z) Parameter (nmax=5) Dimension u(nmax), y1(nmax) C-tst Dimension y5(nmax) Data Zero /0.0d0/, One /1.0d0/, Two /2.0d0/ Data RMax /15.0d0/, dr /0.075d0/ C C Table E6.1 C Do n = 1,nmax Write(*,'(/,1x,79("-"),/," Table E6.1 for n = ",i2,/, $ 1x,79("-"))') n Do l = 0,n-1 Call STO_ULK (n,l,u) If (l+1.eq.2) then Write(*,'(10x," l = ",i2,15x,5f12.5)') l,(u(i),i=1,n-l) else if (l+1.eq.3) then Write(*,'(10x," l = ",i2,27x,5f12.5)') l,(u(i),i=1,n-l) else if (l+1.eq.4) then Write(*,'(10x," l = ",i2,39x,5f12.5)') l,(u(i),i=1,n-l) else if (l+1.eq.5) then Write(*,'(10x," l = ",i2,51x,5f12.5)') l,(u(i),i=1,n-l) else Write(*,'(10x," l = ",i2,3x,5f12.5)') l,(u(i),i=1,n-l) end If end Do end Do write(*,*) C C Profiles C iop = 1 Z = one ! atomic number C iu = 10 ju = 20 ku = 30 Do n = 1,nmax iu = iu + 1 ju = ju + 1 ku = ku + 1 write(*,'(" Profiles of Hydrogenic and Slater-type", $ " Orbitals for n =",i2," are written on unit",i3)') n, iu zeta = z/float(n) ! orbital exponent zetamod = zeta * float(2*n-1) C r = - dr do while (r.lt.rmax) r = r + dr C C Hydrogenic Atomic Orbitals Do l = 0, n-1 y1(l+1) = HAO (n,l,z,r,iop) end Do C C Slater-type Orbital, with zeta = Z/n y2 = STO (n,zeta,r,iop) C C Slater-type Orbital, with zeta = Z*(2n-1)/n y3 = STO (n,zetamod,r,iop) C C Slater-type Orbital, with zeta = Z y4 = STO (n,z,r,iop) C C HAO as a linear combination of STOs C (values to be tested against y1) C-tst Do l = 0, n-1 C-tst Call STO_ULK (n,l,u) C-tst y5(l+1) = 0.0d0 C-tst do k=1,n-l C-tst y5(l+1) = y5(l+1) + u(k) * STO(l+k,zeta,r,iop) C-tst end do C-tst end Do C C Write out radial amplitude profiles C write(iu,'(14f12.8)') r, (y1(i),i=1,n), y2, y3, y4 C-tst$ , (y5(i),i=1,n) C C Write out squared radial amplitude profiles C write(ju,'(14f12.8)') r, (y1(i)**2,i=1,n), y2**2, $ y3**2, y4**2 C C Write out radial density profiles C r2 = r*r Do l = 0, n-1 y1(l+1) = r2 * y1(l+1)**2 end Do y2 = r2 * y2**2 y3 = r2 * y3**2 y4 = r2 * y4**2 write(ku,'(14f12.8)') r, (y1(i),i=1,n), y2, y3, y4 C end do end Do End C ------------------------------------------------------------------ Double Precision Function STO (n,z,r,iop) C ------------------------------------------------------------------ C C Return the radial part of a Slater-type orbital C C n ..... principal quantum number (0, 1, 2, ...) C z ..... orbital exponent C r ..... radial distance C iop ... 0 unnormalized C 1 normalized C C ------------------------------------------------------------------ Implicit Double Precision (a-h,o-z) Data One /1.0d0/, Two /2.0d0/ C STO = (r**(n-1)) * exp(-z*r) C C Normalization constant C if (iop.eq.1) then nn = 2*n STO = STO * sqrt( ((Two*z)**(nn+1)) / Fact(nn) ) end If C C Return End C ------------------------------------------------------------------ Double Precision Function HAO (n,l,z,r,iop) C ------------------------------------------------------------------ C C Return the radial part of a hydrogenic atomic orbital C C n ..... principal quantum number (0, 1, 2, ...) C l ..... azimuthal quantum number (0, 1, 2, ..., n-1) C z ..... atomic number C r ..... radial distance C iop ... 0 unnormalized C 1 normalized C C ------------------------------------------------------------------ Implicit Double Precision (a-h,o-z) Data Zero /0.0d0/, One /1.0d0/, Two /2.0d0/ C alpha = z / float(n) ar = alpha*r tar = two*ar s = zero do k = 0, n-l-1 s = s + (tar**(k+l)) * ((-one)**k)/ $ ( fact(n-l-k-1) * fact(l+l+k+1) * fact(k) ) end do HAO = s * fact(n+l) * exp( -ar ) C C Normalization constant C if (iop.eq.1) then CN = ((two*alpha)**3) * fact(n-l-1) / $ ( float(2*n) * fact(n+l) ) HAO = HAO * sqrt( CN ) end If C Return End C ------------------------------------------------------------------ Subroutine STO_ULK (n,l,u) C ------------------------------------------------------------------ C C Return the coefficients required to express the radial part of an C hydrogenic wavefunction as a linear combination of the radial part C of Slater-type orbitals C C n ... principal quantum number (0, 1, 2, ...) C l ... azimuthal quantum number (0, 1, 2, ..., n-1) C z ... orbital exponent C u ... coefficients C C ------------------------------------------------------------------ Implicit Double Precision (a-h,o-z) Data One /1.0d0/, Two /2.0d0/ Dimension u(*) C UA = float(2*n) * fact(n+l) * fact(n-l-1) C j = 0 Do i = 0, n-l-1 j = j + 1 UB = sqrt( UA * fact(2*l + 2*i + 2) ) UD = fact(n-l-i-1) * fact(2*l+i+1) * fact(i) * float(2*n) u(j) = (-One)**i * UB/UD end Do C Return End C ------------------------------------------------------------------ Double Precision Function Fact (N) C ------------------------------------------------------------------ C C The Factorial of a non-negative integer N, denoted by N!, is the C product of all positive integers less than or equal to N. C ------------------------------------------------------------------ Implicit Double Precision (A-H,O-Z) Data One /1.0D0/ C Fact = One If (N.lt.1) return Do I=1,N Fact = Fact * Float(I) end Do C Return End The last step is to use GRACE [LL1"Grace (plotting tool)",Wikipedia, The Free Encyclopedia, LL2GRACE website] to read the block-data files generated by the fortran code and to arrange the data interactively until the most satisfactory representation is obtained.
|
The graphs of Figure E6.1 show that each time the quantum number n
increases, an additional node is created in the radial wavefunction.
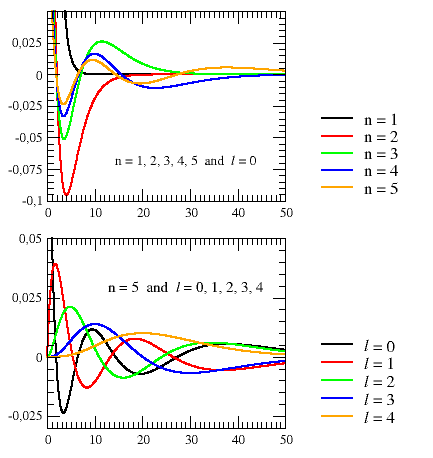
This is made more clear in the upper graph of Figure E6.2, which shows the
ns radial wavefunctions Rn,s(r) of the
hydrogen atom. They differ from the
ns orbitals ψn,s(r,θ,φ) by
a constant angular factor, the spherical harmonic Y00 =
1/√4π. Therefore the ns orbitals
are all spherically symmetrical and, like the radial functions, they have
n − 1 radial nodes (spherical shells on which the wavefunction
equals zero). Each time the quantum number ℓ is increased (lower graph of Figure E6.2), one of the spherical nodes disappears again. It is replaced by a planar node that goes through the nucleus. In the ground state of the hydrogen atom, n = 1, the radial wavefunction is nodeless (i.e. all positive). Its maximum is at r = 0, i.e. the point in space with the highest (joint) probability density of finding the electron is actually inside the nucleus! This is true for all the ns orbitals, whose maximum value is at the origin of the radial coordinate. Therefore, only ℓ = 0 electrons have a finite (joint) probability density at the nucleus. |
At this point it is appropriate to spend a few words trying to overcome some misunderstanding about the position of the electron in the hydrogen atom. The statement that “the probability density of the electron's position in a ns orbital is maximal at the origin” refers to the Born rule, [LL5Born, M. “The statistical interpretation of quantum mechanics”, Nobel lecture, December 11, 1954.] which postulates that the probability density of finding a particle in some region is proportional to the square of the modulus of the wave function, e.g.
| ρ1s(r,θ,φ) = |ψ1s(r,θ,φ)|2 = |R1,0(r)|2 |Y0,0(θ,φ)|2 = Z3 π e−2Z r | (E6.21) |
This is a joint probability density, where the word “joint”
stresses the fact that it is linked to the occurrence of a triple of values,
one for each of the three variables r, θ, φ.
When a joint probability density is multiplied by an infinitesimal volume
element (which in polar spherical coordinates is dV =
r2 sinθ dr
dθ dφ) gives the probability that the values
of the three variables r, θ, φ fall in that
infinitesimal volume element, i.e. the probability of finding the electron
there. This probability is infinitesimal, even if the volume element dV
is positioned at the origin, where the probability density is maximal.
Integrating the probability over a finite volume V yields the
electronic charge contained in V and, if V is extended to all
space, the total charge that amounts to 1e is retrieved, so satisfying
the normalization condition.
Thus, the probability of finding the electron in an infinitesimal volume
element can be written as
| ρn,s(r,θ,φ) r2 sinθ dr dθ dφ = |ψn,s(r,θ,φ)|2 r2 sinθ dr dθ dφ | (E6.22) |
The use of spherical-polar coordinates is convenient because people are used to think of an atom as some sort of fuzzy ball of electrons around a small, central, heavy nucleus, and extend such a mental picture even to bonded atoms in molecules and crystals. In this case, the ns orbitals of the hydrogen atom are spherically-symmetrical (i.e. do not depend on θ and φ), so that integration of eq. (E6.22) over θ and φ gives 4π, the total solid angle of a sphere:
| ρn,s(r) dr = |ψn,s(r)|2 r2 dr π ∫ 0 sinθ dθ 2π ∫ 0 dφ = 4π |ψn,s(r)|2 r2 dr | (E6.23) |
Eq. (E6.23) introduces a “marginal” probability density, namely the “radial” probability density:
| ρn,s(r) = 4π |ψn,s(r)|2 r2 = 4π |Rn,0(r)|2 |Y0,0|2 r2 = |Rn,0(r)|2 r2 | (E6.24) |
Eq. (E6.24) can be generalized for all the permissible values of the quantum numbers n and ℓ, thanks to the form of the wave functions (ψn,ℓ,m or χn,ℓ,m) that are defined as the product of the orthonormalized real spherical harmonics, Yℓ,m(θ,φ), with a radial function, Rn,ℓ(α; r) or Rn(ζ; r):
| ρn,ℓ(r) = |Rn,ℓ(r)|2 r2 π ∫ 0 2π ∫ 0 |Yℓ,m(θ,φ)|2 sinθ dθ dφ = |Rn,ℓ(r)|2 r2 | (E6.25) |
where the double integral expresses the normalization condition of the real spherical harmonics and is equal to one.
Rather than considering the probability of finding the electron in one particular small element of space, the radial density is associated to the probability of finding the electron in a spherical shell of space of infinitesimal thickness, 4π r2 dr (cf eq. E6.23).[N4Volume of a spherical shell of infinitesimal thickness] The plot of radial probability passes through zero at r = 0 since the surface area, 4π r2, the surface area of a sphere of zero radius is zero. As the radius of the sphere is increased, the surface area of the spherical shells increases. However, it increases more rapidly with increasing r than the joint electron probability density decreases, so that the radial probability has a maximum at rmax = a0, which is the Bohr radius for the n = 1 orbit (cf first graph in Figure E6.3). Thus more of the electronic charge is present at a distance a0, out from the nucleus than at any other value of r.
Where is the electron in a hydrogen atom? http://spiff.rit.edu/classes/phys314/lectures/hyd_probs/hyd_probs.html Most probable point for finding an electron in the 1s orbital of a Hydrogen atom https://chemistry.stackexchange.com/questions/84726/most-probable-point-for-finding-an-electron-in-the-1s-orbital-of-a-hydrogen-atom http://www.falstad.com/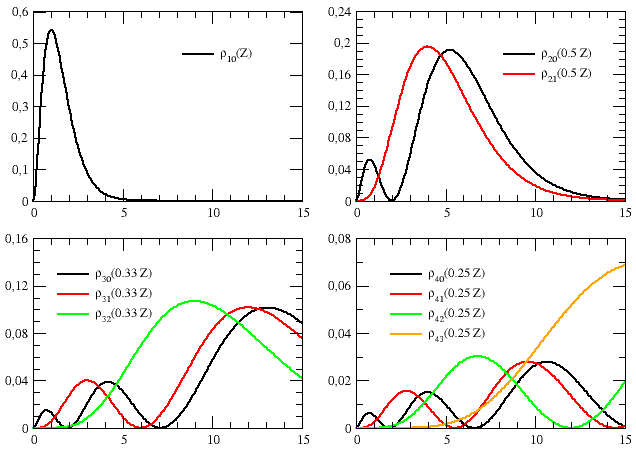
| <r>n.ℓ = +∞ ∫ 0 r ρn,ℓ(r) dr | (E7.1) |
| <rm>n.ℓ = +∞ ∫ 0 rm ρn,ℓ(r) dr | (E7.2) |
| <rm>n.ℓ = +∞ ∫ 0 rm+2 |Rn,ℓ(r)|2 dr = (n − ℓ − 1)! 2n (n + ℓ)! (2 α)3 +∞ ∫ 0 rm+2 (2αr)2ℓ [L2ℓ+1n−ℓ−1(2αr)]2 e−2αr dr | (E7.3) |
| L2ℓ+1n−ℓ−1(s) = n−ℓ ∑ q=1 (−s)q−1 tq | (E7.4) |
| tq = (n + ℓ)! [ (n − ℓ − q)! (q + 2ℓ)! (q − 1)! ]−1 (q = 1, 2, 3, …, n−ℓ) | (E7.5) |
|
<rm>n.ℓ =
nm−1
(n − ℓ − 1)!
(n + ℓ)!
2m+1 Zm
(
n−ℓ
∑
q=1
tq2
+∞
∫
0
s2ℓ+2q+m e−s
ds
+
2
n−ℓ
∑
q=2
q−1
∑
p=1
(−1)p+q tptq
+∞
∫
0
s2ℓ+p+q+m e−s
ds
)
= nm−1 (n − ℓ − 1)! (n + ℓ)! 2m+1 Zm ( n−ℓ ∑ q=1 tq2 (2ℓ + 2q + m)! + 2 n−ℓ ∑ q=2 q−1 ∑ p=1 (−1)p+q tptq (2ℓ + p + q + m)! ) | (E7.6) |
| <rm>n.n−1 = nm (2n + m)! (2n)! 2m Zm | (E7.7) |
| <r>10 = 3 2 Z, <r>21 = 5 Z, <r>32 = 21 2 Z, <r>43 = 18 Z, … | (E7.8) |
| <r2>10 = 3 Z2, <r2>21 = 30 Z2, <r2>32 = 126 Z2, <r2>43 = 360 Z2, … | (E7.9) |
|
t12 (2ℓ + 2 + m)! =
4 (n − 1)2 (2n + m − 2)! t22 (2ℓ + 4 + m)! = (2n + m)! = (2n + m) (2n + m − 1) (2n + m − 2)! − 2t1t2 (2ℓ + 3 + m)! = − 4 (n − 1) (2n + m − 1)! = − 4 (n − 1) (2n + m − 1) (2n + m − 2)! |
| <rm>n.n−2 = nm−1 (2n + m − 2)! [2n + m(m + 3)] (2n − 2)! 2m+1 Zm | (E7.10) |
| <r>20 = 6 Z, <r>31 = 25 2 Z, <r>42 = 21 Z, … | (E7.11) |
| <r2>20 = 42 Z2, <r2>31 = 180 Z2, <r2>42 = 504 Z2, … | (E7.12) |
| <r>n,ℓ = 3n2 − ℓ(ℓ + 1) 2Z | (E7.13) |
| <r2>n,ℓ = n2 [ 5 n2 − 3 ℓ(ℓ + 1) + 1 ] 2 Z2 | (E7.14) |
The standard deviation, i.e. the square root of the variance, of the electron's distribution in the radial direction, σr;n,ℓ, has the following expression:
| σr;n,ℓ = √<r2>n,ℓ − <r>n,ℓ2 = √ n2 (n2 + 2) − ℓ2 (ℓ + 1)2 2 Z | (E7.15) |
An alternative solution formula
for the integral in eq. (E7.3) is reported in the literature.[9Poh-Aun Lee, Seng-Huat Ong, H. M. Srivastava
Intern. J. Computer Math. 2001, 78, 303-321,
equation 18., LL3ResearchGate
online resource: “Some Integrals of the Products of Laguerre
Polynomials”.]
Setting ω =
nm−1
(n − ℓ − 1)!
(n + ℓ)!
2m+1 Zm
, eq (E7.3) can be rewritten as
|
ω−1 <rm>n.ℓ
=
+∞
∫
0
s2ℓ+m+2
[L2ℓ+1n−ℓ−1(s)]2
e−s ds = (2ℓ + m + 2)! n−ℓ−1 ∑ q=0 ( m + 1 n − ℓ − q − 1 )2 ( 2ℓ + m + q + 1 q ) |
Intern. J. Computer Math. 2001, 78, 303-321, equation 18., LL3ResearchGate online resource: “Some Integrals of the Products of Laguerre Polynomials”.] Developing the formula yields
|
ω−1
<rm>n.ℓ =
(
(m + 1)!
(n − ℓ − 1)!
(ℓ + m − n + 2)!
)2
(2ℓ + m +2)! + ( (m + 1)! (n − ℓ − 2)! (ℓ + m − n + 3)! )2 (2ℓ + m + 3)! 1! + ( (m + 1)! (n − ℓ − 3)! (ℓ + m − n + 4)! )2 (2ℓ + m + 4)! 2! + … + ( (m + 1)! (n − ℓ − q − 1)! (m + ℓ − n + q + 2)! )2 (2ℓ + m + q + 2)! q! + … + ( (m + 1)! m! )2 (n + ℓ + m)! (n − ℓ − 2)! + (n + ℓ + m + 1)! (n − ℓ − 1)! |
Using Slater-type orbitals. If eq. (E7.2) is written in terms of STOs, it yields
| <rm>n = +∞ ∫ 0 rm+2 |Rn(r)|2 dr = (2ζ)2n+1 (2n)! +∞ ∫ 0 r2n+m e− 2 ζ r dr = (2n + m)! (2n)! (2ζ)m | (E7.16) |
where the last equality is justified by eq. (E2.5). Eq. (E7.16) can be rewritten explicitly for cases where m = 1 or 2:
| <r>n = 2n + 1 2ζ | (E7.17) |
| <r2>n = (2n + 1) (2n + 2) 4 ζ2 | (E7.18) |
The standard deviation, i.e. the square root of the variance, of the electron's distribution in the radial direction, σr;n, has the following expression:
| σr;n = √<r2>n − <r>n2 = √ 2n + 1 2 ζ | (E7.19) |
From eqs (E7.17) and (E7.19) it follows that the relative error on the average distance of the electron from the nucleus is σr;n <r>n = 1 √ 2n + 1 , which for n = 1 is equal to 57.7%
The numerical values of first and second moments with the associated standard deviation, calculated by means of eqs (E7.13-15) and (E7.17-19), are compared in Table E7.1.
| n,ℓ | exact hydrogenic orbitals | Slater-type orbitals | ||||
|---|---|---|---|---|---|---|
| Z <r>n,ℓ | Z2 <r2>n,ℓ | Z σr;n,ℓ | ζ <r>n | ζ2 <r2>n | ζ σr;n | |
| 1,0 | 1.5 | 3 | 0.87 | 1.5 | 3 | 0.87 |
| 2,0 | 6 | 42 | 2.45 | 2.5 | 7.5 | 1.12 |
| 2,1 | 5 | 30 | 2.24 | |||
| 3,0 | 13.5 | 207 | 4.97 | 3.5 | 14 | 1.32 |
| 3,1 | 12.5 | 180 | 4.87 | |||
| 3,2 | 10.5 | 126 | 3.97 | |||
| 4,0 | 24 | 648 | 8.49 | 4.5 | 22.5 | 1.50 |
| 4,1 | 23 | 600 | 8.43 | |||
| 4,2 | 21 | 504 | 7.94 | |||
| 4,3 | 18 | 360 | 6.00 | |||
| d [r Rn,ℓ(r)] dr = 0 | (E8.1) |
| d [r R1s(r)] dr = d dr ( 2 √Z3 e−Z r ) = 2 √Z3 e−Z r (1 − Z r) = 0 ⇒ rmax = 1 Z | (E8.2) |
For the 2s state of hydrogen, the condition for a maximum is
| d [r R2s(r)] dr = d dr ( √2 Z3 4 (2r − Z r2) e−Z r/2 ) = √2 Z3 8 (Z2 r2 − 6 Z r + 4) e−Z r/2 = 0 ⇒ rmax = 3 ± √5 Z | (E8.3) |
The two possible solutions indicate the presence of two humps in the radial probability density for the 2s orbital (cf the upper-right graph of Figure E6.3).
For the 2p state of hydrogen, a similar analysis yields,
| d [r R2s(r)] dr = d dr ( √6 Z5 12 r2 e−Z r/2 ) = √6 Z5 24 (4 r − Z r2) e−Z r/2 = 0 | (E8.4) |
with the obvious roots rmin = 0 and rmax = 4 Z. Thus, the most probable distances of the electron from the nucleus for the 1s and 2p states are the same predicted by the simple Bohr model for the radius of the first and second orbits. As in the case of the ground state, the most probable value of r in the 2s and 2p states is lower than the average value.
As usual, it is worth to compare the results for the hydrogenic orbitals with those that can be obtained using Slater-type orbitals:
| d [r Rn(r)] dr = d dr ( √ (2ζ)2n+1 (2n)! rn e−ζ r ) = √ (2ζ)2n+1 (2n)! rn−1 (n − ζ r) e−ζ r = 0 | (E8.5) |
with the obvious roots rmin = 0 and rmax = n ζ (cf the graph on the right in Figure E6.4). Again the value of rmax is lower than the average value and the following relationship holds:
| rmax,n = 2 n 2 n + 1 <r>n | (E8.6) |
Exercise: Find the moment generating function of an exponential random variable r with parameter ζ, e.g. the radial density function of the gound state of the hydrogen atom .
| Mr(s) = E(es r) = +∞ ∫ 0 es r ρ(r) dr = γ3 2 +∞ ∫ 0 e−(γ−s)r r2 dr = γ3 (γ − s)3 |
| dn d sn Mr(s) = dn d sn [γ (γ − s)−1]3 = n! 2 (γ − s)n |
| dn d sn Mr(s)❘s=0 = n! 2 γn. |
| ⟨rn⟩ ≡ E(rn) = +∞ ∫ 0 rn ρ(r) dr = γ3 2 +∞ ∫ 0 e−γ r rn+2 dr = n! 2 γn. |
Exercise: Use an 1s-GTO, as a trial function, to find an upper bound to the exact ground state energy of the hydrogen atom .
| ❘Φ⟩ = ( 2α π )3⁄4 e−α r2 | (t) |
|
⟨Φ❘Φ⟩ =
(
2α
π
)3⁄2
+∞
∫
0
e−2α r2
r2 dr
π
∫
0
sinθ dθ
2π
∫
0
dφ
= ( 2α π )3⁄2 ⋅ π½ 4 (2 α)3/2 ⋅ 4 π = 1 | (u) |
|
E =
⟨
Φ❘−
1
2
d2
d
r2 −
1
r
d
d
r
−
Z
r
❘Φ
⟩
=
⟨
Φ❘− 2
α2 r2 + 3 α −
Z
r
❘Φ
⟩
= 4 π ( 2α π )3⁄2 ( − 2 α2 +∞ ∫ 0 e−2α r2 r4 dr + 3 α +∞ ∫ 0 e−2α r2 r2 dr − Z +∞ ∫ 0 r e−2α r2 dr ) = 4 π ( 2α π )3⁄2 [ 3 16 ( π 2 α )1⁄2 − Z 4 α ] = 1 2 ( 3 α − 8 Z √ α 2 π ) | (v) |
Polyanin, A. D., Chernoutsan, A. I., Eds.
Taylor & Francis Group: New York, 2011.] +∞ ∫ 0 r2n e−γ r2 dr = (2 n − 1)!! 2n+1 γn ( π γ )1⁄2 = (2 n)! 22n+1 n! γn ( π γ )1⁄2 and +∞ ∫ 0 r2n+1 e−γ r2 dr = n! 2 γn+1 , have been used. The optimal value of the exponent α is obtained by solving the equation d E d α = 0, i.e.
| 3 − 4 Z √ 2 π α = 0 ⇒ α = 8 Z2 9 π = 0.28294246 Z2 | (w) |
“Modern Quantum Chemistry - Introduction to Advanced Electronic Structure Theory”
Macmillan Publishing Co., Inc., New York, 1982, p. 33.] Finally, the normalization constant becomes
| ( 2α π )3⁄4 = 8 ( Z 3 π )3⁄2 = 0.27649271 Z3/2 | (x) |
|
ρ1s(r) =
64
27
(
Z
π
)3
r2 exp
(
−
16 Z2
9 π
r2
)
| (y) |
Exercise: Write the wave function files (.wfn) for the hydrogen atom using both Slater and Gaussian basis sets.
Name H Run Type SinglePoint Method ROHF Basis Set Slater
SLATER 1 MOL ORBITALS 1 PRIMITIVES 1 NUCLEI
H (CENTRE 1) 0.00000000 0.00000000 0.00000000 CHARGE = 1.0
CENTRE ASSIGNMENTS 1
TYPE ASSIGNMENTS 1
EXPONENTS 1.0000000E+00
MO 1 OCC NO = 1.00000000 ORB. ENERGY = -0.5
0.56418958E+00
END DATA
THE SCF ENERGY = -0.500000000000 THE VIRIAL(-V/T)= 2.00000000
2) Gaussian basis set (a single 1s orbital, according to eq.D?):
Name H Run Type SinglePoint Method ROHF Basis Set STO-1G
GAUSSIAN 1 MOL ORBITALS 1 PRIMITIVES 1 NUCLEI
H (CENTRE 1) 0.00000000 0.00000000 0.00000000 CHARGE = 1.0
CENTRE ASSIGNMENTS 1
TYPE ASSIGNMENTS 1
EXPONENTS 0.2829425E+00
MO 1 OCC NO = 1.00000000 ORB. ENERGY = -0.42441369
0.27649271E+00
END DATA
THE SCF ENERGY = -0.424413690000 THE VIRIAL(-V/T)= 2.00000000
References
- “Exact Gaussian Expansions of Slater-Type Atomic
Orbitals”
Gomes, A. S. P.; Custodio, R. J. Comput. Chem. 2002, 23, 1007-1012. - Szabo, A.; Ostlund, N. S. “Modern Quantum Chemistry − Introduction to Advanced Electronic Structure Theory” Macmillan Publishing Co., Inc., New York, 1982, p. 33. ISBN 0-02-949710-8.
- “A Concise Handbook of Mathematics, Physics, and Engineering Sciences”; Polyanin, A. D., Chernoutsan, A. I., Eds.; Taylor & Francis Group: New York, 2011.
- A. V. Arecchi, T. Messadi, and R. J. Koshel, “Field Guide to Illumination”, SPIE Press, Bellingham, WA, 2007, p. 2. ISBN: 9780819467683.
- Leonard I. Schiff, “Quantum Mechanics”. McGraw-Hill, 1968, Chapter 16, pp. 88-99. ISBN-13: 9780070856431. ISBN-10: 0070856435.
- Paul A. Tipler; Gene Mosca; “Physics for Scientists and Engineers: Extended Version”. W. H. Freeman, 2003, Section 36-4, p. 1181. ISBN-13: 9780716743897. ISBN-10: 0716743892.
- David A. B. Miller, “Quantum Mechanics for Scientists and Engineers”. Cambridge University Press, 2008, Chapter 10, pp. 259-278. ISBN-13: 9780521897839. ISBN-10: 0521897831.
- Milton Abramowitz and Irene A. Stegun, eds. “Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables”. United States Government Printing Office, 1972, § 22.3, p. 775. ISBN-13: 9780318117300. ISBN-10: 0318117304.
- “Some Integrals of the Products of Laguerre
Polynomials”
Lee, P.-A.; Ong, S.-H.; Srivastava, H. M. Intern. J. Computer Math. 2001, 78, 303-321, equation 18. DOI: 10.1080/00207160108805112 - Einstein, A.; Infeld, L. “The Evolution of Physics” Cambridge University Press, 1971, p. 106, ISBN 0-521-09687-1. Internet Archive: accessed February 24, 2019.
Notes
- Probability amplitude.
In classical physics a wave describe "the motion of something which is not
matter, but energy propagated through matter".[last1Albert Einstein, Leopold Infeld, “The Evolution
of Physics”
Cambridge University Press, 1971, p. 106.] Not all waves require a medium, as electromagnetic waves and gravitational waves can travel through a vacuum. Waves are characterized by properties like the wavelength, the frequency, and the amplitude. The intensity of light or sound is found to be proportional to the amplitude of the associated wave. “When Schrödinger first discovered the correct laws of quantum mechanics, he wrote an equation which described the amplitude to find a particle in various places. This equation was very similar to the equations that were already known to classical physicists − equations that they had used in describing the motion of air in a sound wave, the transmission of light, and so on.”[LL4“The Feynman Lectures on Physics”, Online Edition, Vol. III, Ch. 3: “Probability Amplitudes”] Then Max Born postulated that the probability of finding a particle in some region is proportional to the square of the modulus of the wave function (Born rule).[LL5Born, M. “The statistical interpretation of quantum mechanics”, Nobel lecture, December 11, 1954.] So the term (probability) amplitude can be used for the wave function. - Volume element in spherical coordinates.
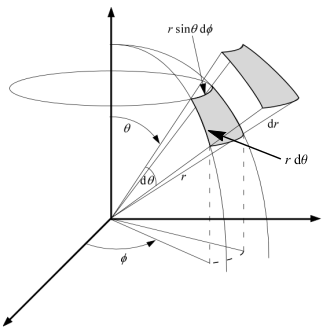
Working in spherical coordinates, the diagram on the left enable us to calculate the infinitesimal volume element as
The volume of a solid is always given by the integration of the infinitesimal volume element dV over the entire solid. We cover a sphere of radius R if we let r ∈ [0,R], θ ∈ [0,π], and φ ∈ [0,2π]. The volume of the sphere is then given bydV = dr ⋅ r dθ ⋅ r sinθ dφ = r2 sinθ dr dθ dφ V = ∫ sphere dV = R ∫ 0 r2 dr π ∫ 0 sinθ dθ 2π ∫ 0 dφ
= [ 1 3 r3 ]R0 ⋅ [−cosθ ]π0 ⋅ [φ ]2π0
= 4 π R3 3 - Surface area element in spherical coordinates.
The diagram of the previous note [N1] also enables us
to calculate the infinitesimal area elements when we integrate over only
two of the spherical coordinates. When we integrate over the surface of
a sphere, we only vary θ and φ by dθ
and dφ, respectively, and we do not vary r at all. The
corresponding area element is given in the diagram of the previous note as
The surface area of a sphere of radius R is obtained by direct integration over the entire sphere, letting θ ∈ [0,π] and φ ∈ [0,2π] while using the spherical coordinate area element R2 sinθ dθ dφ,dA = r2 sinθ dθ dφ A = ∫ sphere dA = R2 π ∫ 0 sinθ dθ 2π ∫ 0 dφ
= R2 ⋅ [−cosθ ]π0 ⋅ [φ ]2π0
= 4 π R2 - Solid angle. A solid angle is the 3 dimensional analog of
an ordinary angle. It is related to the surface area of a sphere in the same
way an ordinary angle is related to the circumference of a circle.
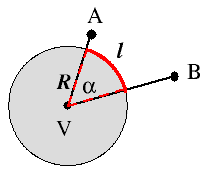
A plane angle, α, is made up of the lines that from two points A and B meet at a vertex V: it is defined by the arc length (red curve in the figure on the left) of a circle subtended by the lines and by the radius R of that circle (dotted red lines),[4A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2.] as shown on the left. The dimensionless unit of plane angle is the radian. The value in radians of a plane angle α is the ratio
of the length ℓ of a circular arc to its radius R. It follows that the plane angle subtended by the full circle measures 2π radians.α = ℓ R
The infinitesimal element of a solid angle Ω is given by dΩ = dA R2 , where dA is the surface area element in spherical coordinates defined in [N2Surface area element in spherical coordinates.], so thatA solid angle is made up from all the lines that from a closed curve meet at a vertex: it is defined by the surface area A of a sphere subtended by the lines and by the radius R of that sphere,[4A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2.] as shown in the Figure on the right. The dimensionless unit of solid angle is the steradian. The measure in steradians of a solid angle Ω is the area A on the surface of a sphere of radius R divided by the radius squared,
and is independent of the particular value of the chosen radius. Because the area A of the entire spherical surface is 4πr2, the definition implies that a sphere subtends 4π steradians at its center. By the same argument, the maximum solid angle that can be subtended at any point is 4π steradians.Ω = A R2 ,
Concisely, the solid angle Ω subtended by a surface S is defined as the surface area Ω of a unit sphere covered by the surface’s projection onto the sphere.[L4Weisstein, E. W. "Solid Angle,"
From MathWorld - A Wolfram Web Resource.] That’s a very complicated way of saying the following: You take a surface, like the bright red circle in the figure on the right. Then you project the edge of the circle (but in general it could be any closed curve) to the center of a sphere of any radius R. The projection intersects the sphere and forms a surface area A. Then you calculate the surface area of your projection and divide the result by the radius squared. That's it.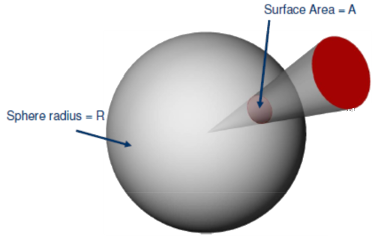
is equal to the differential surface area on the unit sphere. Its integral over the solid angle of all space being subtended is the surface area of the unit sphere, which is 4 π steradians.[L4Weisstein, E. W. "Solid Angle,"dΩ = sinθ dθ dφ
From MathWorld - A Wolfram Web Resource., L5Wikipedia contributors, "Solid angle,"
Wikipedia, The Free Encyclopedia.]Ω = ∫ Ω dΩ = π ∫ 0 sinθ dθ 2π ∫ 0 dφ = [−cosθ ]π0 ⋅ [φ ]2π0 = 4 π - Volume of a spherical shell of infinitesimal thickness.
Consider two spheres, both centered in the origin: the inner with radius
r and the outer with radius r + dr. To compute the
volume of the spherical shell between their two surfaces, proceed as follows:
where, in the last equality, the terms drn with n > 1 have been neglected. In a different way, using differentiation of volume V = 4 3 π r3 w.r.t. radius, you get area (rate of change of volume is area)dV = 4 3 π (r + dr)3 − 4 3 π r3 = 4 3 π (r3 + 3r2dr + 3r dr2 + dr3 − r3 (N1.1) = 4 3 π (3r2dr + 3r dr2 + dr3) ≈ 4 π r2dr d V d r = 4 π r2 ⇒ d V = 4 π r2 d r (N1.2) - U-substitution. Many pages on the Web
are dedicated to the method of integration by substitution, also known as the
u-substitution. Among these, we remember the page of Wikipedia.[L6Wikipedia contributors, "Integration by
substitution,"
Wikipedia, The Free Encyclopedia.] The u-substitution is also described in some pages of the PAMoC manual, in particular in section 4.7 of the chapter on "Charge Distributions"“Transformation of the Random Vector Variable”
PAMoC Manual: Charge Distributions, Section 4.7, and in sections 1.5 and 2.3 of the chapter on "Methods of Numerical Integration" “Transformation of coordinates” (section 1.5)
“Radial integral and transformation of the radial coordinate” (section 2.3)
PAMoC Manual: Methods of Numerical Integration.. - Remember that ∫ f '(x) ef(x) dx = ef(x) + C.
- Real Spherical Harmonics. Orthonormalized Laplace’s
spherical harmonics are defined as
where Pℓm(cosθ) is the associate Legendre polynomial of degree ℓ and order m ( ℓ ≥ 0 and −ℓ≤ m ≤ ℓ). Spherical harmonics are single-valued, smooth (infinitely differentiable), complex functions of two variables, θ and φ, indexed by two integers, ℓ and m, and form a complete set of orthonormal functions:Yℓm(θ,φ) = (−1)m Nℓm Pℓm(cosθ) eimφ (N7.1)
This normalization is used in quantum mechanics because it ensures that probability is normalized, i.e. π ∫ 0 |Yℓm(Ω)|2 dΩ = 1, with dΩ = sinθ dθ and normalization factor:2π ∫ 0 dφ π ∫ 0 Yℓm(θ,φ) Yℓ'm'*(θ,φ) sinθ dθ = δℓℓ' δmm' (N7.2)
The real forms of the spherical harmonics, also known as tesseral harmonics, are obtained by taking the linear combinations [Yℓm(θ,φ) ± Yℓ−m(θ,φ)]/√2. They are defined as followsNℓm = √ 2ℓ + 1 4 π ⋅ (ℓ − |m|)! (ℓ + |m|)! (N7.3)
whereYℓm(θ,φ) = Mℓm Uℓm(θ,φ) (N7.4)
are the un-normalized tesseral harmonics, and Mℓm is the normalization factorUℓm(θ,φ) = Pℓ|m|(cosθ) { cos(|m|φ), m ≥ 0 sin(|m|φ), m < 0
(N7.5)Mℓm = Nℓm √ 2 − δ|m|,0 = √ 2ℓ + 1 4 π ⋅ (2 − δ|m|,0) ⋅ (ℓ − |m|)! (ℓ + |m|)! (N7.6)
Links
- Euler integrals. Encyclopedia of Mathematics. URL: https://www.encyclopediaofmath.org/index.php?title=Euler_integrals&oldid=25725 (accessed January 16, 2019.
- Wikipedia contributors, "Gamma function," Wikipedia, The Free
Encyclopedia.
https://en.wikipedia.org/w/index.php?title=Gamma_function&oldid=878188592 (accessed January 16, 2019). - Weisstein, Eric W. "Gamma Function." From MathWorld −
A Wolfram Web Resource.
http://mathworld.wolfram.com/GammaFunction.html (accessed January 16, 2019). - Weisstein, Eric W. "Reflection Relation." From MathWorld −
A Wolfram Web Resource.
http://mathworld.wolfram.com/ReflectionRelation.html (accessed January 17, 2019). - Weisstein, Eric W. "Leibniz Integral Rule." From MathWorld −
A Wolfram Web Resource.
http://mathworld.wolfram.com/LeibnizIntegralRule.html (accessed January 17, 2019). - Weisstein, Eric W. "Multifactorial." From MathWorld −
A Wolfram Web Resource.
http://mathworld.wolfram.com/Multifactorial.html (accessed January 19, 2019). - Wikipedia contributors, "Particular values of the gamma function,"
Wikipedia, The Free Encyclopedia.
https://en.wikipedia.org/w/index.php?title=Particular_values_of_the_gamma_function&oldid=874045399 (accessed January 18, 2019). - Wikipedia contributors, "Spherical harmonics." Wikipedia, The Free
Encyclopedia.
https://en.wikipedia.org/wiki/Spherical_harmonics (accessed October 3, 2018). - Wikipedia contributors, "Table of spherical harmonics." Wikipedia,
The Free Encyclopedia.
https://en.wikipedia.org/wiki/Table_of_spherical_harmonics (accessed October 3, 2018). - Weisstein, Eric W. “Spherical Harmonic.” From
MathWorld − A Wolfram Web Resource.
http://mathworld.wolfram.com/SphericalHarmonic.html (accessed October 3, 2018). - Weisstein, Eric W. “Solid Angle.” From
MathWorld − A Wolfram Web Resource.
http://mathworld.wolfram.com/SolidAngle.html (accessed October 4, 2018). - Wikipedia contributors, “Solid angle.” Wikipedia,
The Free Encyclopedia.
https://en.wikipedia.org/wiki/Solid_angle (accessed October 4, 2018). - Wikipedia contributors, “Integration by substitution.”
Wikipedia, The Free Encyclopedia.
https://en.wikipedia.org/wiki/Integration_by_substitution (accessed October 12, 2018). - Mark E. Tuckerman, “The Schrödinger equation for the
hydrogen atom and hydrogen-like cations”, 2011-10-26.
(Lecture 8: CHEM-UA 127: Advanced General Chemistry I)
http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_8/node5.html (accessed October 31, 2018). - “Solid Angle”. Optipedia: SPIE Press books opened for
your reference. Excerpt from “Field Guide to Illumination” by
Angelo V. Arecchi, Tahar Messadi, R. John Koshel.[4A. V. Arecchi, T. Messadi, and R. J. Koshel
“Field Guide to Illumination”
SPIE Press, Bellingham, WA, 2007, p. 2.]
http://spie.org/publications/fg11_p02_solid_angle (accessed November 12, 2018). - Rudi Winter,
“Solving Schrödinger's equation for the hydrogen atom”
http://users.aber.ac.uk/ruw/teach/237/hatom.php (Accessed February 18, 2019). - “GRACE: GRaphing, Advanced Computation and Exploration of
data”. Online resource:
http://plasma-gate.weizmann.ac.il/Grace/.
Accessed February 7, 2019.
- “Some Integrals of the Products of Laguerre
Polynomials”
Lee, P.-A.; Ong, S.-H.; Srivastava, H. M. Intern. J. Computer Math. 2001, 78, 303-321, equation 18.
Online resource: https://www.researchgate.net/publication/233359915_Some_Integrals_of_the_products_of_laguerre_polynomials. Accessed February 13, 2019. - “The Feynman Lectures on Physics”, Online Edition,
Vol. III, Ch. 3: “Probability Amplitudes”.
www.feynmanlectures.caltech.edu/III_03.html (accessed Febrary 24, 2019). - Born, M. “The statistical interpretation of quantum
mechanics”. Nobel lecture, December 11, 1954.
Online resource (PDF):
www.nobelprize.org/uploads/2018/06/born-lecture.pdf. Accessed
February 24, 2019.
“Again an idea of Einstein's gave me the lead. He had tried to make the duality of particles - light quanta or photons - and waves comprehensible by interpreting the square of the optical wave amplitudes as probability density for the occurrence of photons. This concept could at once be carried over to the ψ-function: |ψ|2 ought to represent the probability density for electrons (or other particles).”